The first SERRS multiplexing from labelled oligonucleotides in a microfluidics lab-on-a-chip
Frances T.
Docherty
a,
Paul B.
Monaghan
b,
Ruth
Keir
a,
Duncan
Graham
a,
W. Ewen
Smith
*a and
J. M.
Cooper
b
aDept. of Pure and Applied Chemistry, University of Strathclyde, Cathedral Street, Glasgow, UK G1 1XL. E-mail: w.e.smith@strath.ac.uk; Fax: 0141 552 0876; Tel: 0141 548 2615
bDept. of Electronics and Electrical Engineering, Oakfield Avenue, University of Glasgow, Glasgow, UK G12 8LT. E-mail: jmcooper@elec.gla.ac.uk; Fax: 0141 330 6010; Tel: 0141 330 4931
First published on 14th November 2003
Abstract
The first simultaneous detection of three dye-labelled oligonucleotides in a microfluidics chip by SERRS is reported.
The growing need for accurate and fast methods of DNA detection in the post genomic era has generated the development of a number of new platforms for sample analysis. Two of the most popular approaches have been the use of microarrays of immobilized probes and microfluidic chips. Both approaches require a detection technique to be used with the platform which has a high enough sensitivity for such small analyte volumes. The techniques of choice tend to be mass spectrometry or fluorescence spectroscopy. Here we propose surface enhanced resonance Raman scattering (SERRS) as an alternative spectroscopy for detection. SERRS and fluorescence have comparable sensitivities but one main advantage of SERRS is that the peaks in the spectra are much narrower and are more easily resolved. This, in principle, opens up the potential for the simultaneous detection of multiple labels to a degree not possible with fluorescence, thus providing an enabling technology for the more complex assays required since the completion of the human genome map. Also, when using SERRS both fluorophores and nonfluorophores are suitable so that a more extensive and simpler labelling chemistry can be employed.
We have reported several studies on the use of SERRS for DNA analysis1–4 and described the use of SERRS for multiplex genotyping of the cystic fibrosis gene.5 However, in all of the previous work the samples have been analysed in microtitre plates. This current study now describes the first use of SERRS to simultaneously detect three labeled oligonucleotides in a microfluidic chip. We believe that this is the first step in the development of a fast and highly sensitive multiple recognition system which could be used in a variety of situations including genetic sequencing and detection of explosives.
Fig. 1 shows a schematic view of the chip design (left) and a scanning electron micrograph of part of the chip (right). SERRS requires a metal surface for enhancement and a coloured label for a resonance contribution. In this case silver colloid and water are introduced into channels A and B respectively. Pre-mixed analyte and spermine enter through channel C. Spermine is required to aggregate the colloid and neutralise the phosphate backbone in the oligonucleotide. The chip was designed in this manner so that the colloid and aggregating agent were not mixed together before the introduction of the analyte, to avoid over-aggregation of the colloid before the SERRS data is collected at point X.
 | ||
| Fig. 1 Schematic diagram of the chip design and SEM image of part of a PDMS chip. | ||
In our previous report of the detection of a model analyte in a microfluidics chip by SERRS,6 a glass chip was used. In our current work we now investigate the first use of polymer chips made of polydimethylsiloxane (PDMS) for SERRS analysis. Rather than fabricating a glass chip, a silicon mould was made onto which the PDMS could be poured and cured. This provides a cheap and quick way of producing numerous chips, much more easily than those made in glass, greatly increasing their dispensability and reducing risk of cross contamination.
To make the mould a silicon wafer was cleaned in piranha solution (7 : 1 conc. H2SO4 : 30% H2O2) for 15 min, rinsed in deionised water and then dried in nitrogen. The wafer was then spin coated with AZ4562 photoresist at 4000 rpm for 30 s (∼6 µm thick film), and was baked on a hotplate at 90 °C for 2 min. Once cooled, the acetate mask pattern was transferred to the photoresist by exposure on a mask aligner. The substrate was developed in 4 : 1 v/v water/AZ A400K developer solution for 2 min and dried in nitrogen. The exposed silicon was then etched to a depth of 100 µm on an STS inductively coupled plasma reactive ion etch machine. The remaining photoresist was removed in acetone. The silicon was then silanized with dichloromethylsilane. To fabricate the chips, PDMS Sylgard Elastomer 184 was mixed in a 10 : 1 w/w base to curing agent ratio and poured over the silicon mould. This was left for 1 h to allow the sample to degas, and was then baked at 70 °C for 1 h and was finally removed from the mould.
A cover plate was required to attach tubing to the microfluidics cells. This was manufactured by drilling 1 mm holes in a glass slide. Graphite ferrules providing mechanical support were bonded over these holes with a cyanoacrylate adhesive. Once cured, fused silica capillary was inserted into the holes via the ferrules and held in place with a few drops of araldite. The microstructured PDMS was then placed on the glass base plate allowing fluidic connections.
Fig. 2 shows the SERRS acquired in a microfluidics chip from two separate oligonucleotide sequences described in Table 1. We have previously reported the single and multiplex SERRS spectra from these analytes recorded in a microtitre plate,2 which therefore provided a good model system for evaluating the use of microfluidics for this type of analysis. The spectra were acquired using a Renishaw 2000 Raman Microprobe with a charge-coupled device (CCD) spectrometer. The excitation was provided by a Spectra-Physics Model 2020 argon-ion laser with a wavelength of 514.5 nm and 3 mW of power at the source. The fluids were pumped through the chip at a flow rate of 26 µl s−1 using pressure driven flow provided by a syringe pump attached at the outlet channel at point D. When it was obvious that the main channel was filled with the analyte and aggregated colloid mixture the pump was stopped and the SERRS spectrum acquired. The spectra were collected at point X using a 20× objective and an acquisition time of 1 s.
 | ||
| Fig. 2 SERRS from rhodamine and HEX labelled oligonucleotides and from a mixture of both analytes, recorded in a microfluidics chip. | ||
| Oligo | Disease state detectable | Basic priming sequence (5′ to 3′) | Dye label |
|---|---|---|---|
| 1 | Cystic fibrosis | GTG CTG CAG GTG TAA ACT TGT ACC AG | HEX |
| 2 | Cystic fibrosis | GTG CTG CAG GTG TAA ACT TGT ACC AG | R6G |
| 3 | Escherichia coli | 5C 5C 5C 5C 5C 5C 5C 5C CCC CAC TGC TGC CTC CCG TAG | FAM |
| 4 | Escherichia coli | 5C 5C 5C 5C 5C 5C 5C 5C GAA GGT CCC CCT CTT TGG TCT TG | TET |
| 5 | Escherichia coli | 5C 5C 5C 5C 5C 5C 5C 5C ATA AAT CGC CAT TCG TTG ACT AC | Cy3 |
It is clear that well-defined spectra were obtainable from the dye labels in PDMS cells. The spectra are identical to those acquired from bulk samples in microtitre plates. This indicates that there is adequate mixing of the analyte and aggregated colloid for effective surface enhancement of the Raman signal, although the laminar flow in the chip is not conducive to mixing of the reagents. The absence of any additional peaks also suggests that PDMS does not have a strong SERRS signal which could interfere with that of the analyte at these wavenumbers, making disposable PDMS chips suitable for SERRS.
Fig. 2 also shows the SERRS spectrum recorded in a chip from a mixture of the two labelled oligonucleotides. In a similar manner to the single analytes, the two oligonucleotides were premixed with spermine and sucked through inlet C, whilst colloid and water were sucked through inlets A and B respectively. It is clear that both analytes can be easily recognised in the spectrum from the mixture. In particular the peak at 1629 cm−1 is obviously due to HEX and that at 1647 cm−1 can be assigned to rhodamine.
A further example of SERRS multiplexing recorded from a microfluidics chip is shown in Fig. 3. In this case a further three oligos, each with different sequences and different dye labels, have been simultaneously detected. Table 1 gives the sequences which are for E. coli variants and therefore provide a good example of how the simultaneous detection of dye-labelled oligonucleotides could be applied to clinical analysis. In a further step towards clinical analysis, the spectra were acquired using a S2000 Ocean Optics portable spectrometer with a 532 nm laser rather than the laboratory based Renishaw system.
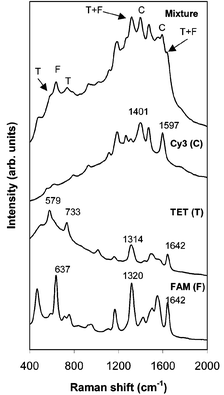 | ||
| Fig. 3 SERRS spectra recorded by a portable spectrometer from single labelled oligonucleotides which have E. coli sequences. The multiplex spectrum from a mixture of all three labels is also shown and each analyte can be identified. | ||
The SERRS from the individual analytes and from a mixture of all three of them are shown in Fig. 3. Although the spectrum from the mixture is complex the contributions from each of the three analytes are readily distinguishable in the multiplex spectrum and can be readily deconvoluted using currently available software. A number of peaks have been highlighted to show the contributions from all three dye labelled oligonucleotides. The peak in the multiplex spectrum at 632 cm−1 can be assigned to the FAM label, 1397 and 1592 cm−1 to Cy3 and 584 and 736 cm−1 to TET. The peak at 1319 cm−1 and shoulder at 1638 cm−1 will have contributions from both FAM and TET. Fig. 3 also shows that although the portable Raman spectrometer has a poorer resolution than the laboratory based Renishaw instrument, it is still good enough for the individual dye labels to be identified in the multiplex spectrum.
In conclusion we have demonstrated the simultaneous detection by SERRS of up to three labelled oligonucleotides in a microfluidics chip. The ability to analyse these samples in a chip will lead to cheaper and more environmentally friendly analysis since smaller sample volumes will be required. We have also shown that PDMS is a suitable fabrication material for the chip for SERRS analysis. The cheap and disposable nature of these chips will reduce risks of cross-contamination and save time cleaning chips. The narrow SERRS signals provide the capability to enable the simultaneous detection of multiple analytes and the ability to use a low-resolution portable spectrometer will also dramatically reduce the cost of such systems. Our demonstration of the simultaneous detection of three oligonucleotides with E. coli sequences is a vital step towards a device for clinical analysis using this technology.
Notes and references
- D. Graham, W. E. Smith, A. M. T. Linacre, C. H. Munro, N. D. Watson and P. C. White, Anal. Chem., 1997, 69, 4703 CrossRef CAS.
- D. Graham, B. J. Mallinder and W. E. Smith, Angew. Chem., Int. Ed., 2000, 39, 1061 CrossRef CAS.
- D. Graham, B. J. Mallinder and W. E. Smith, Biopolymers (Biospec.), 2000, 57, 85 Search PubMed.
- D. Graham, R. Brown and W. E. Smith, Chem. Commun., 2001, 1002 RSC.
- D. Graham, B. J. Mallinder, D. Whitcombe and W. E. Smith, ChemPhysChem, 2001, 746 CrossRef CAS.
- R. Keir, E. Igata, M. Arundell, W. E. Smith, D. Graham, C. McHugh and J. M. Cooper, Anal. Chem., 2002, 74, 1503 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
