Continuous synthesis of CdSe–ZnS composite nanoparticles in a microfluidic reactor
Hongzhi
Wang
,
Xianying
Li
,
Masato
Uehara
,
Yoshiko
Yamaguchi
,
Hiroyuki
Nakamura
*,
Masaya
Miyazaki
,
Hazime
Shimizu
and
Hideaki
Maeda
*
Micro-space Chemistry Lab, National Institute of Advanced Industrial Science and Technology (AIST), 807-1 Shuku, Tosu, Saga 841-052, Japan
First published on 2nd December 2003
Abstract
ZnS-coated CdSe composite particles have been continuously synthesized in a microfluidic reactor. By using this system, CdSe particles and a ZnS coating can be produced in sequence, and the particle size and layer thickness can be directly adjusted by the residence time. It demonstrated that the continuous synthesis in the microreactor was a simple and efficient way to prepare composite particles with different structures and determine the optimized experimental conditions.
Chemical synthesis in microreactors is a novel way of conducting chemistries in a highly controlled fashion with better yields at a reduced overall effort.1,2 Continuous synthesis is believed to be one advantage of microreactor technology, which means the possibility of running up to 24 h per day and doing analyses on line. Especially, a multi-step continuous synthesis in a microreactor was expected to provide better quality functional products with improved economics for complicated reactions. A few references have described the continuous preparation of nanoparticles with homogeneous components and structures such as CdS, CdSe, TiO2 using the microchannel system.3–6 The results highlight the flexibility of such a microfluidic system for efficiently collecting kinetic data and fine-tuning the desired physical properties. In these studies, the experimental conditions tried to remain uniform throughout the total process, such that the temperature variation was controlled to be as low as possible, and no new reactant was added to the reaction process. However, for composite nanoparticles, such as core-shell structure nanoparticles,7–10 different experimental conditions for the core and shell are usually needed, and in some cases, a new reactant has to be added during the reaction. So far, to the best of our knowledge, no report can be found about the composite nanoparticles continuously synthesized in a microreactor. In addition, realizing a multi-step continuous synthesis in a microreactor means more extensive application of this technique in industry. Here we selected the semiconductor composite nanoparticle CdSe–ZnS as the system to demonstrate a multi-step continuous synthesis system because it has been well established that the particle properties can be determined by their UV and luminescence spectra.11–16
Se powder was added to trioctyl phosphine (TOP) to prepare a TOP-Se stock solution. Meanwhile, Cd(CH3COO)2 was added to stearic acid and heated at 130 °C. Trioctyl phosphine oxide (TOPO) was then added under a nitrogen flow. After the solution was cooled to below 100 °C, it was mixed with TOP-Se according to the molecular ratio of 1Cd : 5Se to make the CdSe raw material solution. 0.5 mmol of diethylzinc and 0.5 mmol of bis(trimethylsilyl) sulfide were dissolved in 3 mL of TOP. They were then mixed with 3 mL of melted TOPO to make the ZnS raw materials solution. The multi-step continuous system was set up as depicted in Fig. 1. There were three sections whose functions included the CdSe synthesis, mixing (with ZnS raw materials), and ZnS coating. The reactant solution flowed in the microchannel (inner diameter: 200 µm) supplied by the microcapillary and a ceramic micromixer, which was used to add the ZnS raw materials into the CdSe crude solution. Two oil baths supply different temperatures for the nucleation and growth of the CdSe and ZnS, respectively. In this system, the microcapillary showed more flexibility than the chip-based microreactor to distribute the reactant residence time in the three sections, and to provide the different temperatures.
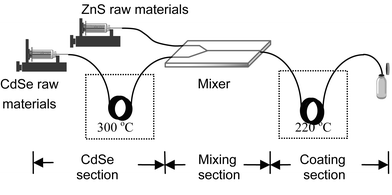 | ||
| Fig. 1 Schematic illustration of the multi-step continuous synthesis system. | ||
Fig. 2 shows the absorption and luminescence spectra of product obtained in the CdSe section and final outlet in this continuous system. For this sample, the CdSe section capillary length was designed to be 10 cm, the mixing section (mixer channel plus capillary) length was 85 cm, and the ZnS coating section was 20 cm. If the flow rate is 100 µl min−1, the residence time for the CdSe synthesis is 2 sec, for mixing, it is 8 sec, and for coating, it is 2 sec. The UV spectra (Fig. 2a) revealed CdSe nanoparticles can be obtained first in the CdSe section of this continuous system. After the CdSe particles flow through the mixing and coating section, its absorption peak red shifted about 10 nm. To test whether the CdSe core nanoparticles grow during the coating process, the CdSe core solution prepared as in ref. 4 was injected into a microcapillary at 220 °C with different residence times. The CdSe absorption peak did not significantly change in a short residence time (less than 5 min) at 220 °C, which means that the CdSe cores grew only slightly without the ZnS raw material addition during the synthesis procedure. Therefore, the absorption red shift of the final product revealed the forming of the ZnS coating, which was often due to partial leakage of the exciton into the ZnS matrix, or the closing together of the electronic levels that accompanies a size increase.15,16Fig. 2b shows that the fluorescence intensity of the final product increased nearly five times compared with the CdSe section product if the flow volume is 100 µl min−1, but decreased with a slower flow volume. The variation in the fluorescence intensity can be explained by the change in the ZnS layer thickness because the different residence time in the ZnS coating section would result in a different ZnS layer thickness. The optimized ZnS layer thickness was also found by Talapin et al. who controlled the layer thickness by the concentration of the ZnS raw materials,13 and they believed that a 1.6 monolayer was the best (a monolayer is about 3.1 Å according to the distance between the consecutive planes along the [002] axis in the bulk wurtzite ZnS). In our experiments, CdSe particles with different ZnS monolayers can be easily obtained by changing the flow rate or capillary lengths, which demonstrated that the multi-step continuous synthesis in the microreactor was a simple and efficient way to control the composite particle structure and find the optimized experimental conditions.
 | ||
| Fig. 2 (a) UV-Vis absorption spectra of product obtained in CdSe portion and final outlet. The flow volume was 100 µl min−1. (b) Luminescence spectra of uncoated CdSe from CdSe portion and coated particles from the outlet with different flow volumes. Label denotes the flow volume of two syringes for the CdSe and ZnS raw materials. The two flow volumes were kept to be the same. Uncoated CdSe was obtained under the flow volume of 100 µl min−1. | ||
Our experiment also showed the importance of mixing for the multi-step continuous synthesis in a microreactor. If the design of the mixing section (mixer channel plus capillary) length is 30 cm, and the flow volume is 100 µl min−1, the residual time for mixing is only 2.7 sec. The UV spectra of the product from the outlet showed an absorption peak for CdSe, but the fluorescence spectra just showed the same intensity as our previous TOPO-capped CdSe particle. No evidence indicated that ZnS had coated the CdSe particles. It is well known in microchannels having dimensions on the order of 100 µm, mass transfer is dominated by laminar flow, and thus mixing is principally driven by diffusion.17–21 The effectiveness of the mixing technique is based on the diffusion equilibration time, t. In our microreactor system, to obtain sufficient mixing, the reactant residence time T in the mixing portion should be greater than or equal to the equilibration time t, which means that the ZnS raw material solution can have enough time to mix with the CdSe particles before the coating process. The residence time T in the mixing section is determined by the flow rate and the capillary plus micromixer channel length. Our experimental results showed that a longer capillary is a simple and effective way to get sufficient mixing in the multi-step continuous synthesis.
In summary, ZnS-coated CdSe particles have been obtained using a total continuous method in a microfluidic reactor. The results not only displayed the same tendency in the UV and luminescence spectra as the conventional method in a flask, but also exhibited the obvious advantage of the microreactor technology in conveniently controlling the structure and properties of the nanoparticles. Through this system, CdSe particles and a ZnS coating can be produced in sequence, and particle size and layer thickness can be directly adjusted by the flow rate. At the same time, this experiment revealed the importance of the micromixer for the prefigured reaction. A more efficient mixer was expected to optimize and miniaturize this system in the future. In addition, the result showed the possibility of operating a more complicated reaction in the microreactor, which means a greater potential for its application.
References
- W. Enrfeld, V. Hessel, and H. Lowe, Microreactors, New Technology For Modern Chemistry, Wiley-VCH, Weinheim 2000 Search PubMed.
- T. Schwalbe, V. Autze and G. Wille, Chimia, 2002, 56, 636–646 CAS.
- H. Z. Wang, H. Nakamura, M. Uehara, M. Miyazaki and H. Maeda, Chem. Commun., 2002, 1462–1463 RSC.
- H. Nakamura, Y. Yamaguchi, M. Miyazaki, H. Maeda, M. Uehara and P. Mulvaney, Chem. Commun., 2002, 2844–2845 RSC.
- J. B. Edel, R. Fortt, J. C. deMello and A. J. deMello, Chem. Commun., 2002, 1136–1137 RSC.
- E. M. Chan, R. A. Mathies and A. P. Alivisatos, Nano Lett., 2003, 3, 199–201 CrossRef CAS.
- Q. Liu, Z. Xu, J. A. Finch and R. Egerton, Chem. Mater., 1998, 10, 3936–3940 CrossRef CAS.
- T. Ung, L. Liz-Marzan and P. Mulvaney, Langmuir, 1998, 14, 3740–3748 CrossRef CAS.
- M. Ohmori and E. Matijevic, J. Colloid Interface Sci., 1992, 150, 594–597 CrossRef CAS.
- H. Z. Wang, H. Nakamura, K. Yao, M. Uehara, S. Nishimura, H. Maeda and E. Abe, J. Am. Ceram. Soc., 2002, 85, 1937–1940 CAS.
- C. F. Hoener, K. A. Allan, A. J. Bard, A. Campion, M. A. Fox, T. E. Mallouk, S. E. Webber and J. M. White, J. Phys. Chem., 1992, 96, 3812–3817 CrossRef CAS.
- M. Hines and P. Guyot-Sinonnest, J. Phys. Chem., 1996, 100, 468–471 CrossRef CAS.
- D. V. Talapin, A. L. Rogach, A. Komowski, M. Haase and H. Weller, Nano Lett., 2001, 1, 207–211 CrossRef CAS.
- M. Kuno, J. K. Lee, B. O. Dabbousi, F. V. Mikulec and M. G. Bawend, J. Chem. Phys., 1997, 106, 9869–9881 CrossRef CAS.
- B. O. Dabbousi, J. Rodriguez-Viejo, F. V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen and M. G. Bawendi, J. Phys. Chem. B, 1997, 101, 9463–9475 CrossRef CAS.
- X. Peng, M. C. Schlamp, V. Kadavanich and A. P. Alivisatos, J. Am. Chem. Soc., 1997, 119, 7019–7029 CrossRef CAS.
- G. H. Seong and R. M. Crooks, J. Am. Chem. Soc., 2002, 124, 13360–13361 CrossRef CAS.
- T. J. Johnson, D. Ross and L. E. Locascio, Anal. Chem., 2002, 74, 45–51 CrossRef CAS.
- A. D. Stroock, S. K. W. Dertinger, A. Ajdari, I. Mezic, H. A. Stone and G. M. Whitesides, Science, 2002, 295, 647–651 CrossRef CAS.
- B. H. Weigl and P. Yager, Science, 1999, 283, 346–347 CrossRef.
- P. J. A. Kenis, R. F. Ismagilov and G. M. Whitesides, Science, 1999, 285, 83–85 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
