Spatially separated bimetallic cocatalysts on hollow-structured TiO2 for photocatalytic hydrogen generation†
Ping
She
 a,
Jun-sheng
Qin
a,
Jun-sheng
Qin
 ab,
Heng
Rao
ab,
Heng
Rao
 ab,
Buyuan
Guan
ab and
Jihong
Yu
ab,
Buyuan
Guan
ab and
Jihong
Yu
 *ab
*ab
aState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun 130012, P. R. China. E-mail: jihong@jlu.edu.cn
bInternational Center of Future Science, Jilin University, 2699 Qianjin Street, Changchun 130012, P. R. China
First published on 30th March 2020
Abstract
Efficient charge separation and light harvesting of photocatalysts (e.g., TiO2) are the key issues to be considered in the design of solar-energy conversion systems. In particular, the charge separation of noble metal-decorated TiO2 materials could be greatly improved via decreasing the size of noble metal particles (NPs). Furthermore, designing specific morphologies such as hollow structures can improve light harvesting ability. Herein, a hybrid hollow TiO2 with spatially separated bimetals (Pd@TiO2@Au) was prepared, which demonstrated enhanced charge separation. By choosing zeolite as the sacrificial substrate, ultrasmall Pd NPs and Au NPs were decorated in the inner and outer shells of hollow TiO2, respectively. The separated bimetals could pull the photoexcited electrons away from the surface of TiO2 for more efficient charge separation. The as-prepared Pd@TiO2@Au catalyst exhibited a superior photocatalytic H2 evolution rate up to 272.3 μmol h−1, which was higher than most of the TiO2-based photocatalysts.
Introduction
Nowadays, the problem of energy shortage and environmental pollution is becoming more and more serious in the world.1–5 Hydrogen (H2) as a kind of clean fuel has drawn intensive attention for its superior energy density and environmental friendliness.1–3 Converting solar energy into H2 by photocatalytic water splitting is one of the most efficient ways for H2 production. A large number of semiconductor photocatalysts including titanium dioxide (TiO2), CdS, Ta3N5, ZnO and g-C3N4 have been widely used for photocatalytic H2 generation.6,7 Among them, TiO2 has been considered as one of the most promising photocatalysts owing to its low price, high safety and superior chemical stability.8–11 However, the fast recombination of the photogenerated electron–hole pairs strongly hinders the photocatalytic hydrogen production. Recently, noble metal nanoparticles (NPs) (e.g., Au, Pt, and Ag NPs) were decorated on photocatalysts to enhance the catalytic properties.12–15 In particular, the bimetal decorated TiO2 (such as Pd–Au, Pt–Cu, and Au–Pt) can exhibit further improved photocatalytic efficiency compared to the monometal decorated ones.16–20 Among them, most of the bimetals were randomly decorated outside the TiO2 with the inner interface not being used. Thus, the spatially separated bimetals on the inner and outer surfaces of TiO2 are expected to be able to enhance the photocatalytic properties by fully utilizing the photo-induced charges on the interface of excited TiO2.On the other hand, recent investigations showed that the size of noble metal NPs has a great influence on the photocatalytic properties of the nanohybrids.6,21 It has been demonstrated that the smaller noble metal NPs can induce a lower Fermi level, which can cause improved charge transfer and an elevated separation rate of electron–hole pairs.21 Unfortunately, it is hard to obtain ultrasmall noble metal clusters since they are prone to aggregation. Nowadays, zeolites have been widely used for the confinement synthesis of ultrasmall metal NPs and even sub-nanometric metallic clusters, in which the nanospace of zeolites can restrict the growth of the metals.22–24 Also, the ordered microporous structures of zeolites can eliminate the aggregation of metal species and thus improve the catalytic properties and stability.25
Furthermore, it has been demonstrated that the nanostructures of the hybrids of noble metal–TiO2 play an important role in improving the photocatalytic properties.11,26–30 Notably, the nano hybrids with a yolk–shelled hollow morphology can induce higher photocatalytic properties.30 On the one hand, the hollow structure can enhance light utilization efficiency by realizing repeated light reflection and refraction; on the other hand, the hollow structure can increase the surface-to-volume ratio, which can provide more active reaction sites and enhance mass transfer.
Herein, we designed a hollow-structured TiO2 with spatially separated bimetallic NPs decorated in the core and outer shells, respectively. In detail, the hollow TiO2 sphere was obtained by choosing silicalite-1 (MFI) zeolite with embedded ultrasmall Pd NPs as a supporting substrate followed by etching of the zeolite. The TiO2 exhibited a hollow structure and the ultrasmall Pd NPs were decorated inside the hollow TiO2 sphere. Then, Au NPs working as the cocatalyst were decorated on the outer surfaces of the TiO2 shell. The obtained sandwich-like hollow-structured Pd@TiO2@Au with spatially separated cocatalysts can significantly enhance the photocatalytic properties of TiO2 by combining the surface plasmon resonance (SPR) effect of Au NPs and the smaller size effect of Pd NPs. Specifically, Pd@TiO2@Au exhibited a superior photocatalytic H2 evolution rate up to 272.3 μmol h−1. Such performance is far superior to most of the TiO2-based photocatalysts for water-splitting.
Experimental
Materials
Chloroauric acid tetrahydrate (HAuCl4·4H2O, A.R., Shanghai Chemical Factory), tetraethylorthosilicate (TEOS) (Sinopharm Chemical Reagent Co., Ltd), tetrabutyl orthotitanate (TBOT) (98%, Macklin), tetrapropylammonium hydroxide (TPAOH) (25 wt%, Tianjin Guangfu Fine Chemical Research Institute), ethanol (99%, Tianjin Guangfu Fine Chemical Research Institute), palladium chloride (PdCl2, Pd 59%, Aladdin), ethylenediamine (NH2CH2CH2NH2, Tianjin Fuchen Chemical Reagents Factory), ammonia (NH3·H2O, Tianjin Yongsheng Fine Chemical Co., Ltd), sodium hydroxide (NaOH, A.R., Tianjin Yongsheng Fine Chemical Co., Ltd), and deionized water (resistance >18 MΩ cm−1).Preparation of silicalite-1 (S1) zeolite
S1 was prepared by using the hydrothermal method at 170 °C for 4 days, the molar composition of which is 1.0SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4TPAOH
0.4TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35H2O. Specifically, 13 g of TPAOH was mixed with deionized water followed by stirring for 10 min, then 8.32 g of TEOS was added to the above solution. After being stirred for 6 h, the mixture was transferred to a Teflon-lined stainless-steel autoclave and heated at 170 °C for 4 days under static conditions. After washing with deionized water and ethanol three times, the product was then dried at 60 °C. Finally, conventional S1 was obtained by calcinating the above product at 550 °C in air for 8 h.
35H2O. Specifically, 13 g of TPAOH was mixed with deionized water followed by stirring for 10 min, then 8.32 g of TEOS was added to the above solution. After being stirred for 6 h, the mixture was transferred to a Teflon-lined stainless-steel autoclave and heated at 170 °C for 4 days under static conditions. After washing with deionized water and ethanol three times, the product was then dried at 60 °C. Finally, conventional S1 was obtained by calcinating the above product at 550 °C in air for 8 h.
Synthesis of Pd@silicalite-1 (Pd@S1)
Pd@S1 was synthesized using the same method for the preparation of S1 except for the addition of [Pd(NH2CH2CH2NH2)2]Cl2 after the hydrolysis of TEOS.27 After calcination, the product was subsequently reduced with H2 at 400 °C for 2 h. In this way, Pd@S1 was obtained.Preparation of hollow Pd@TiO2, hollow TiO2 and TiO2 NPs
Typically, 150 mg of the above-synthesized zeolite or Pd@S1 was dispersed in 200 mL of absolute ethanol. After adding 0.9 mL of ammonia solution (28 wt%) into the system followed by ultrasonication for half an hour, TBOT (2.0 mL) was added into the solution drop by drop. Then, the mixture was stirred at 45 °C for 24 hours. After washing with deionized water and ethanol several times and calcination at 450 °C for 2 h, Pd@S1@TiO2 NPs were obtained. In order to get hollow structured Pd@TiO2, 50 mg of Pd@S1@TiO2 NPs was treated with 30 mL of NaOH solution (3 M) under stirring for 12 h. After centrifugation and washing with water and ethanol 3 times, hollow Pd@TiO2 was obtained. When it comes to the synthesis of hollow TiO2 NPs, S1 was used to replace Pd@S1. The conventional TiO2 NPs were prepared using the same method for preparing hollow TiO2 except without adding zeolite as the template.Preparation of sandwich-like Pd@TiO2@Au, hollow TiO2@Au, Pd@TiO2@Pt and hollow TiO2@Pt
The sandwich-like Pd@TiO2@Au was synthesized by photo-deposition of the Au NPs on the surfaces of Pd@TiO2. Specifically, the starting gel of sandwich-like Pd@TiO2@Au was obtained by dispersing Pd@TiO2 into HAuCl4 (0.06%) solution and adjusting the pH to 8 (adjusted by 0.1 M NaOH). After irradiation at 200–1100 nm for 20 minutes, Au3+ was converted into Au NPs. In this way, the sandwich-like Pd@TiO2@Au was obtained. The hollow TiO2@Au was obtained by replacing Pd@TiO2 with hollow TiO2. The sandwich-like Pd@TiO2@Pt was synthesized using the same method except for replacing HAuCl4 by H2PtCl6. The hollow TiO2@Pt was prepared by replacing Pd@TiO2 with hollow TiO2.Synthesis of Pd-im/silicalite-1 (Pd-im/S1) and Pd-im@TiO2
For comparison, Pd-im/S1 was obtained using the impregnation method.27 Typically, 0.23 mL of (NH4)2PdCl4 solution (0.28 M) was added into 1 g of S1. After stirring for an hour, the mixture was dried overnight at 80 °C. Finally, Pd-im/S1 was obtained by reducing the dried mixture with hydrogen. Then, Pd-im@TiO2 was prepared using the same method for preparing Pd@TiO2 except for replacing Pd@S1 by Pd-im/S1.Synthesis of Pd–Au-im@TiO2 and Pd–Pt-im@TiO2
Pd–Au-im@TiO2 and Pd–Pt-im@TiO2 were prepared using the impregnation approach. The metal loadings in Pd–Au-im@TiO2 and Pd–Pt-im@TiO2 were controlled to be comparable with those of sandwich-like Pd@TiO2@Au and Pd@TiO2@Pt accordingly. First, Pd@S1 was impregnated with HAuCl4 and H2PtCl6 solutions, respectively. Then, Pd–Au-im@S1 and Pd–Pt-im@S1 were obtained by reducing the above two mixtures with hydrogen at 400 °C for 2 h. Finally, Pd–Au-im@TiO2 and Pd–Pt-im@TiO2 were obtained by growing TiO2 on the outside surface of Pd–Au-im@S1 and Pd–Pt-im@S1, respectively, followed by etching the zeolite with NaOH solution. The methods for the growth of TiO2 and the etching details are the same as those for the preparation of sandwich-like Pd@TiO2@Au.Material characterization
Powder X-ray diffraction was performed on a Rigaku Smart Lab X-ray diffractometer using Cu Kα radiation (λ = 1.5418 Å) in the 2θ range from 4° to 80°. Scanning electron microscopy (SEM) images and energy dispersive X-ray (EDX) spectrometry images were obtained using a JSM-7800F (Japan) electron microscope. The transmission electron microscopy (TEM) images and EDX spectrometry images were obtained using a Tecnai F20 electron microscope. The metal loading was determined using inductively coupled plasma (ICP) analyses on a PerkinElmer Optima 3300 DV ICP instrument. X-ray photoelectron spectroscopy (XPS) was carried out on an ESCALAB 250 spectrometer. UV-vis absorption spectra were obtained using a SHIMADZU UV-2550 spectrophotometer (200–800 nm). The photoluminescence (PL) spectral measurements were conducted on a SHIMADZU RF-5301pc spectrofluorophotometer. The PL lifetime decay curves were plotted using a HORIBA Scientific FluoroMax-4 spectrofluorometer.Photocatalytic measurements
Typically, 10 mg of the photocatalyst was dispersed in 20 mL of an aqueous solution containing 4 mL of methanol, then the solution was transferred into a quartz vessel. After being vacuumed for 20 min to remove the dissolved air, the vessel was irradiated by a 300 W Xenon lamp under simulated solar light (200–1100 nm) with a light intensity of 250 mW cm−2. The gas products generated from photocatalytic water splitting were analyzed periodically using an Agilent 7890A gas chromatograph (GC) with a thermal conductivity detector (TCD).Photocurrent measurements
The electrochemical experiments were performed on a CHI 660E electrochemistry work station in Na2SO4 (0.5 M) solution. Platinum wire and the AgCl/Ag electrode (saturated with KCl) were used as the counter electrode and the reference electrode, respectively. The working electrodes were made by loading the samples on indium-tin oxide (ITO). In detail, 10 mg of the photocatalysts was dispersed in 200 μL of deionized water, and then the obtained slurry was dropped onto ITO to form a square with the area of 1 cm2 followed by drying at 50 °C for 30 min. Following dropping 20 μL of Nafion on the surface of the photocatalysts, the photoelectrodes of hollow TiO2, hollow TiO2@Pt, Pd@TiO2 and Pd@TiO2@Au were obtained. Light illumination was provided by a 300 W Xe lamp with the light intensity of 300 mW cm−2.Results and discussion
The synthesis process of the sandwich-like hollow-structured Pd@TiO2@Au photocatalyst is shown in Fig. 1. First, ultrasmall Pd NPs were obtained by using an MFI type zeolite (silicalite-1) as the host matrix and [Pd(NH2CH2CH2NH2)2]Cl2 as the metal precursor, respectively, using the method developed by our group.31 Specifically, the precursor of Pd NPs was introduced to the hydrothermal crystallization process of silicalite-1 (S-1). After calcination and followed by reduction with H2, the ultrasmall Pd NPs confined in silicalite 1 (Pd@S1) were obtained. Subsequently, Pd@S1 was covered with a layer of amorphous TiO2 after mixing Pd@S1 in tetrabutyl titanate (TBOT) and ammonia solution at 45 °C for 24 h. Following calcination at 450 °C for 2 h, Pd@S1@TiO2 with anatase TiO2 was obtained. Afterwards, Pd@S1@TiO2 was transformed into hollow-structured Pd@TiO2 after being treated with the NaOH solution. In this way, the sandwich-like hollow-structured Pd@TiO2@Au was achieved by photo-reducing HAuCl4 on the outer surface of Pd@TiO2. For comparison, hollow TiO2 and conventional TiO2 NPs were also prepared by using pure S1 as the sacrificial substrate and without adding zeolites, respectively. In addition, Pd-im@TiO2 with larger Pd NPs was achieved by growing TiO2 on the outside surface of Pd-im/S1 (obtained using the impregnation method). In order to further elucidate the effect of spatially separated cocatalysts on the sandwich-like hollow structured Pd@TiO2@Au photocatalyst, Pd–Au-im@TiO2 with both the cocatalysts (Pd and Au NPs) decorated on the inner surface of hollow TiO2 was prepared.As shown in the scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images (Fig. 2 and Fig. S1, S2, ESI†), S1, Pd@S1, Pd@TiO2 and the sandwich-like Pd@TiO2@Au all display the shape of a hexagonal prism with the size of about 200–300 nm. According to the TEM images of Pd@S1 obtained by the in situ process, the ultrasmall Pd clusters (<2 nm) are uniformly located inside the zeolite crystals (Fig. 2a and b). In comparison, the Pd-im/S1 sample prepared by the impregnation method possesses a larger size of Pd NPs (∼10 nm), as shown in Fig. 2c. The TEM image of Pd@S1@TiO2 in Fig. 2d shows that a layer of TiO2 with the thickness of about 35 nm covers the outer surface of Pd@S1. As shown in Fig. 2e and f, both Pd@TiO2 and Pd@TiO2@Au exhibit a hollow structure. Due to the small size, the inner Pd NPs are invisible. The Au NPs in Pd@TiO2@Au decorated on the outer surface of hollow Pd@TiO2 are about 10 nm in size. In contrast, the Pd NPs in the hollow Pd-im@TiO2 (Fig. 2g) are more obvious for their larger size. In addition, the hexagonal S1@TiO2 (Fig. 2h) and hollow TiO2 (Fig. 2i) and conventional TiO2 NPs (Fig. S3, ESI†) were also obtained.
The SEM and TEM elemental mappings of Pd@TiO2 and Pd@TiO2@Au are shown in Fig. 3a–i and Fig. S4 (ESI†), revealing the existence of Pd, Ti, O and Au elements. The Pd, Ti and O elements are distributed uniformly in hollow-structured Pd@TiO2 and Pd@TiO2@Au. Furthermore, it appears that the Au NPs are deposited on the outside surface of hollow TiO2 in sandwich-like Pd@TiO2@Au (Fig. S4, ESI†). According to the inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis, the Au and Pd loading in the sandwich-like hollow-structured Pd@TiO2@Au is 4.89 and 0.62 wt%, respectively.
The as-prepared sandwich-like hollow-structured Pd@TiO2@Au photocatalyst was further characterized using X-ray diffraction (XRD). The as-synthesized S1 and Pd@S1 exhibit the typical diffraction peaks of the MFI zeolite structure (Fig. S5, ESI†).22 For Pd@S1@TiO2, the diffraction peaks belonging to anatase-TiO2 can be obviously seen (Fig. S6, ESI†).29 According to Fig. S6, S7 (ESI†) and Fig. 4a, all the TiO2-containing samples (TiO2 NPS, hollow TiO2, Pd@TiO2, hollow TiO2@Au and Pd@TiO2@Au) show typical diffraction peaks of anatase-TiO2. Besides, the peaks of face-centered-cubic Au can be seen in the XRD profiles of hollow TiO2@Au and Pd@TiO2@Au.29
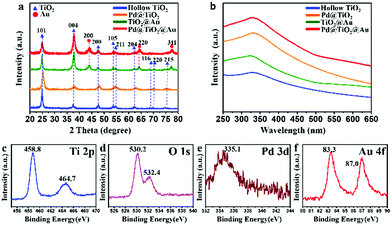 | ||
| Fig. 4 (a) XRD profiles and UV-vis spectra (b) of hollow TiO2, Pd@TiO2, hollow TiO2@Au and sandwich-like Pd@TiO2@Au; (c)–(f) XPS spectra of sandwich-like Pd@TiO2@Au. | ||
The UV-vis spectra in Fig. 4b indicate that the existence of both Pd NPs and Au NPs can enhance the light absorption of hollow TiO2. Furthermore, Pd@TiO2@Au with spatially separated Pd NPs and Au NPs exhibits much higher light absorption efficiency than the hollow TiO2 and monometal cocatalyst decorated ones (Pd@TiO2 and hollow TiO2@Au). Besides, TiO2@Au and Pd@TiO2@Au with decorated Au NPs show much higher light absorption ability in the visible light range, which may be caused by the elevated light absorption due to the SPR effect and the light scattering of Au NPs.27
The X-ray photoelectron spectroscopy (XPS) analyses of sandwich-like Pd@TiO2@Au are shown in Fig. 4c–f. There are two peaks belonging to Ti 2p that appear at around 458.8 and 464.7 eV, indicating the Ti4+ valence state in octahedral coordination with oxygen.32 The signal of O 1s exhibits a peak at 530.2 eV corresponding to the Ti–O bonds in the TiO2 lattice, and the peak located at the shoulder (532.4 eV) is related to the oxygen in the surface hydroxyl groups.33 The peak at 335.1 attributed to Pd 3d5/2 is observed in Pd@TiO2@Au, which is related to the zero-valent Pd NPs.31 The Au 4f spectrum displays two peaks at 83.3 and 87.0 eV with the splitting of 3.7 eV, indicating the metallic nature of Au.34
The photocatalytic H2 evolution activities of sandwich-like Pd@TiO2@Au together with hollow TiO2, Pd@TiO2, and hollow TiO2@Au were investigated under simulated solar light. In the photocatalytic reaction system, methanol (CH3OH) was added as the sacrificial agent to react with the holes. As shown in Fig. 5a–b and Fig. S8 (ESI†), all of the hollow-structured photocatalysts exhibit enhanced hydrogen generation compared to the TiO2 NPs. The H2 evolution rate of hollow TiO2 (14.3 μmol h−1) is about 2 times faster than that of the TiO2 NPs (6.5 μmol h−1 in Fig. S8, ESI†). This phenomenon can be attributed to the elevated light harvesting induced by the hollow structure. Notably, Pd@TiO2@Au with spatially separated bimetallic cocatalysts shows greatly improved photocatalytic properties. The H2 generation rate of Pd@TiO2 and TiO2@Au reaches up to 210.8 and 119.5 μmol h−1, respectively. Significantly, Pd@TiO2@Au gives a H2 generation rate up to 272.3 μmol h−1. For comparison, we also prepared sandwich-like Pd@TiO2@Pt by using Pt to replace Au to further test whether different separated bimetallic cocatalysts on TiO2 could still work (Fig. 5c). The morphologies of Pd@TiO2@Pt, hollow TiO2@Pt and Pt-im/Pd@S1 are shown in Fig. S9 and S10 (ESI†). As expected, Pd@TiO2@Pt with similar types of spatially separated cocatalysts (Pd NPs and Pt NPs) shows an enhanced hydrogen evolution rate (648.6 μmol h−1) compared to TiO2 NPs and hollow TiO2. In addition, Pd@TiO2@Pt gives a higher elevated hydrogen evolution rate than Pd@TiO2 (210.8 μmol h−1) and TiO2@Pt (464.0 μmol h−1). To further demonstrate the effects of the spatially separated cocatalysts on TiO2, Pd–Au-im@TiO2 and Pd–Pt-im@TiO2 with both bi-noble metal NPs decorated within the hollow cavity were also prepared to compare with Pd@TiO2@Au and Pd@TiO2@Pt correspondingly (Fig. 5d). The H2 generation rates of Pd–Au-im@TiO2 and Pd–Pt-im@TiO2 are 159.3 and 264.2 μmol h−1, respectively, which are much lower than that of Pd@TiO2@Au and Pd@TiO2@Pt decorated with the spatially separated bimetallic cocatalyst. Therefore, we can conclude that spatially separated bi-noble metals play an important role in enhancing the photocatalytic efficiency.
 | ||
| Fig. 5 (a) H2 generation over the sandwich-like Pd@TiO2@Au catalyst, hollow TiO2, Pd@TiO2 and hollow TiO2@Au; (b) H2 evolution rates based on the corresponding catalysts in Fig. 5a; (c) H2 generation over the Pd@TiO2@Pt catalyst and the corresponding controls; (d) H2 generation over Pd–Au-im@TiO2 and Pd–Pt-im@TiO2 for comparison with Pd@TiO2@Au and Pd@TiO2@Pt. | ||
On the other hand, the size effect of Pd NPs plays an important role in the photocatalytic efficiency of Pd@TiO2. For comparison, Pd-im@TiO2 with larger sized Pd NPs was also obtained by replacing Pd@S1 with Pd-im/S1 to work as the sacrificial substrate. As shown in Fig. S11 (ESI†), Pd-im@TiO2 shows a lower hydrogen evolution rate (163.5 μmol h−1) than Pd@TiO2 (210.8 μmol h−1). Therefore, the smaller sized Pd NPs synthesized by in situ crystallization of the zeolite can further improve the catalytic properties of TiO2. It is worth mentioning that the hydrogen generation rate of sandwich-like Pd@TiO2@Au (272.3 μmol h−1) with the quantum efficiency of 1.64% (Fig. S12, ESI†) is superior to most of the TiO2-based photocatalysts for photocatalytic hydrogen generation under similar conditions (Table S1, ESI†).34–42
Fig. 6a shows the photocurrent generation performance of hollow TiO2, Pd@TiO2, hollow TiO2@Au and sandwich-like Pd@TiO2@Au. It can be clearly seen that the existence of Pd NPs and Au NPs can improve the photocurrent density. Specifically, the sandwich-like Pd@TiO2@Au with spatially separated cocatalysts exhibits the highest photocurrent density, demonstrating the efficient photoinduced charge separation properties. To further investigate the charge separation properties of the as-prepared photocatalysts, the photoluminescence (PL) emission spectrum was recorded. As shown in Fig. 6b, all the samples exhibit characteristic peaks at around 400 nm. In detail, all the TiO2 hybrids with decorated cocatalysts (Pd NPs or Au NPs) show weaker PL emission compared to hollow TiO2, which may be attributed to the lower Fermi level of Pd NPs and Au NPs.21 Furthermore, the PL emission of Pd@TiO2 is lower than that of hollow TiO2@Au, suggesting that Pd@TiO2 possesses greater charge transfer ability than hollow TiO2@Au. Notably, the sandwich-like Pd@TiO2@Au exhibits the weakest PL emission among all of the samples, further confirming the superior charge separation performance. To better understand the behaviour of the photo-excited charge carriers, the time-resolved fluorescence decay spectra of the as-prepared photocatalysts were also studied (Fig. 6c). According to the bar chart of PL decay time shown in Fig. 6d, the sandwich-like Pd@TiO2@Au displays a longer decay time than the other samples, further indicating the superior charge separation of the photocatalyst with spatially separated bimetals.
The proposed mechanism of photocatalytic hydrogen generation over the sandwich-like Pd@TiO2@Au catalyst is shown in Fig. 7. Upon irradiation by incident light, holes are generated on the valence band (VB) of TiO2 and photo-induced electrons are produced on the conduction band (CB). Subsequently, the photo-induced electrons can be quickly transferred from TiO2 to the cocatalysts (Pd NPs and Au NPs).43,44 In addition, the SPR effect induced by the Au NPs on the outside surface of TiO2 can stimulate an enhanced electronic field, which can not only improve the generation rate of electron–hole pairs, but also facilitate the charge transfer.45 Meanwhile, charge separation can be further improved by the ultrasmall size effect of Pd NPs located on the inner surface of TiO2.46 In this way, more efficient charge transfer and quantum efficiency can be obtained. Moreover, the photo-generated holes on the VB of TiO2 can be used to oxidize the scavenger (CH3OH) to produce H+ ions. Afterward, the H+ ions can combine with the electrons on the CB of TiO2 or cocatalysts to produce H2. Specifically, the spatially separated bimetallic cocatalysts can further enhance the photocatalytic properties of sandwich-like Pd@TiO2@Au due to the enhanced charge separation, as shown in Fig. 6.
Conclusions
A hollow-structured TiO2 decorated with spatially separated bimetallic cocatalysts (Pd@TiO2@Au) was obtained by using a zeolite as the sacrificial substrate. Pd@TiO2@Au exhibits superior photocatalytic hydrogen generation ability based on the factors below: first, the hollow structure of TiO2 can not only enhance light utilizing ability by repeated light reflection and refraction, but also provide more active reaction sites and facilitate mass transfer through the enhanced specific surface area. Second, the spatially separated bimetallic cocatalysts (Pd and Au) can further increase the photocatalytic charge transfer and separation by fully utilizing the photo-induced charges on both the inner and outer surfaces of the excited TiO2. Thirdly, the SPR effect induced by the Au NPs on the outside surface of TiO2 can stimulate an enhanced electronic field, which can not only improve the generation rate of electron–hole pairs, but also facilitate the charge transfer. Fourth, the ultra-small Pd NPs synthesized by the in situ crystallization process of zeolite can further elevate the charge separation efficiency. The facile approach demonstrated here holds great promise for the design of highly efficient photocatalysts for the application of environmental purification and solar-to-hydrogen energy conversion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Key Research and Development Program of China (Grant 2016YFB0701100), the National Natural Science Foundation of China (Grant 21835002 and 21621001), the 111 Project (B17020), the Post Doctoral Innovative Talent Support Program (BX20180122) and the China Postdoctoral Science Foundation (2019M651195) for supporting this work.Notes and references
- J. L. Wu, Z. Y. Zhang, B. K. Liu, Y. R. Fang, L. Wang and B. Dong, UV-Vis-NIR-driven plasmonic photocatalysts with dual-resonance modes for synergistically enhancing H2 generation, Sol. RRL, 2018, 2, 1800039 CrossRef.
- Y. Liu, Z. Y. Zhang, Y. R. Fang, B. K. Liu, J. D. Huang, F. J. Miao, Y. A. Bao and B. Dong, IR-driven strong plasmonic-coupling on Ag nanorices/W18O49 nanowires heterostructures for photo/thermal synergistic enhancement of H2 evolution from ammonia borane, Appl. Catal., B, 2019, 252, 164–173 CrossRef CAS.
- D. P. Dong, C. X. Yan, J. D. Huang, N. Lu, P. Y. Wu, J. Wang and Z. Y. Zhang, An electron-donating strategy to guide the construction of MOF photocatalysts toward co-catalyst-free highly efficient photocatalytic H2 evolution, J. Mater. Chem. A, 2019, 7, 24180–24185 RSC.
- M. Y. Zhang, Y. Qi and Z. Y. Zhang, AgBr/BiOBr nano-heterostructure-decorated polyacrylonitrile nanofibers: a recyclable high-performance photocatalyst for dye degradation under vsible-light irradiation, Polymers, 2019, 11, 1718 CrossRef CAS PubMed.
- P. Zhang, D. Y. Wan, Z. Y. Zhang, G. D. Wang, J. H. Hu and G. S. Shao, RGO-functionalized polymer nanofibrous membrane with exceptional surface activity and ultra-low airflow resistance for PM2.5 filtration, Environ. Sci.: Nano, 2018, 5, 1813–1820 RSC.
- Q. M. Sun, N. Wang, J. H. Yu and J. C. Yu, A hollow porous CdS photocatalyst, Adv. Mater., 2018, 30, 1804368 CrossRef PubMed.
- Y. G. Zhu, Z. Y. Zhang, N. Lu, R. N. Hua and B. Dong, Prolonging charge-separation states by doping lanthanide-ions into {001}/{101} facets-coexposed TiO2 nanosheets for enhancing pho-cmssmark tocatalytic H2 evolution, Chin. J. Catal., 2019, 40, 413–423 CrossRef CAS.
- C. Tang, L. F. Liu, Y. L. Li and Z. F. Bian, Aerosol spray assisted assembly of TiO2 mesocrystals into hierarchical hollow microspheres with enhanced photocatalytic performance, Appl. Catal., B, 2017, 201, 41–47 CrossRef CAS.
- M. Z. Ge, Q. S. Li, C. Y. Cao, J. Y. Huang, S. H. Li, S. N. Zhang, Z. Chen, K. Q. Zhang, S. S. Al-Deyab and Y. K. Lai, One-dimensional TiO2 nanotube photocatalysts for solar water splitting, Adv. Sci., 2017, 4, 1600152 CrossRef PubMed.
- Y. X. Zhao, Y. F. Zhao, R. Shi, B. Wang, G. I. N. Waterhouse, L. Z. Wu, C. H. Tung and T. R. Zhang, Tuning oxygen vacancies in ultrathin TiO2 nanosheets to boost photocatalytic nitrogen fixation up to 700 nm, Adv. Mater., 2019, 31, 1806482 CrossRef PubMed.
- Z. W. Seh, S. H. Liu, M. Low, S. Y. Zhang, Z. L. Liu, A. Mlayah and M. Y. Han, Janus Au–TiO2 photocatalysts with strong localization of plasmonic near-fields for efficient visible-light hydrogen generation, Adv. Mater., 2012, 24, 2310–2314 CrossRef CAS PubMed.
- Z. Q. Liang, Y. C. Guo, Y. J. Xue, H. Z. Cui and J. Tian, 1T-phase MoS2 quantum dots as a superior co-catalyst to Pt decorated on carbon nitride nanorods for photocatalytic hydrogen evolution from water, Mater. Chem. Front., 2019, 3, 2032–2040 RSC.
- J. L. He, S. Li, D. Lyu, D. F. Zhang, X. Wu and Q. H. Xu, Aggregation induced emission enhancement by plasmon coupling of noble metal nanoparticles, Mater. Chem. Front., 2019, 3, 2421–2427 RSC.
- L. Cheng, D. N. Zhang, Y. L. Liao, F. Li, H. W. Zhang and Q. J. Xiang, Constructing functionalized plasmonic gold/titanium dioxide nanosheets with small gold nanoparticles for efficient photocatalytic hydrogen evolution, J. Colloid Interface Sci., 2019, 555, 94–103 CrossRef CAS PubMed.
- S. Q. Kong, X. M. Song, C. W. Zhang, W. Wang and G. C. Yan, Photocatalytic activity and photodegradation rate constant of Au–TiO2 nanofilms by magnetron sputtering, Rare Met. Mater. Eng., 2018, 47, 3316–3320 CrossRef.
- Z. Y. Zhang, S. W. Cao, Y. S. Liao and C. Xue, Selective photocatalytic decomposition of formic acid over AuPd nanoparticle-decorated TiO2 nanofibers toward high-yield hydrogen production, Appl. Catal., B, 2015, 162, 204–209 CrossRef CAS.
- Z. Y. Zhang, A. R. Li, S. W. Cao, M. Bosman, S. Z. Li and C. Xue, Direct evidence of plasmon enhancement on photocatalytic hydrogen generation over Au/Pt-decorated TiO2 nanofibers, Nanoscale, 2014, 6, 5217–5222 RSC.
- H. B. Yu, X. H. Wang, H. W. Sun and M. X. Huo, Photocatalytic degradation of malathion in aqueous solution using an Au–Pd–TiO2 nanotube film, J. Hazard. Mater., 2010, 184, 753–758 CrossRef CAS PubMed.
- Y. Shiraishi, H. Sakamoto, Y. Sugano, S. Ichikawa and T. Hirai, Pt-Cu bimetallic alloy nanoparticles supported on anatase TiO2: highly active catalysts for aerobic oxidation driven by visible light, ACS Nano, 2013, 7, 9287–9297 CrossRef CAS PubMed.
- A. Gallo, M. Marelli, R. Psaro, V. Gombac, T. Montini, P. Fornasiero, R. Pievo and V. Dal Santo, Bimetallic Au–Pt/TiO2 photocatalysts active under UV-A and simulated sunlight for H2 production from ethanol, Green Chem., 2012, 14, 330–333 RSC.
- K. Qian, B. C. Sweeny, A. C. Johnston-Peck, W. X. Niu, J. O. Graham, J. S. DuChene, J. J. Qiu, Y. C. Wang, M. H. Engelhard, D. Su, E. A. Stach and W. D. Wei, Surface plasmon-driven water reduction: gold nanoparticle size matters, J. Am. Chem. Soc., 2014, 136, 9842–9845 CrossRef CAS PubMed.
- T. L. Cui, W. Y. Ke, W. B. Zhang, H. H. Wang, X. H. Li and J. S. Chen, Encapsulating palladium nanoparticles inside mesoporous MFI zeolite nanocrystals for shape-selective catalysis, Angew. Chem., Int. Ed., 2016, 55, 9178–9182 CrossRef CAS PubMed.
- L. C. Liu, U. Diaz, R. Arenal, G. Agostini, P. Concepcion and A. Corma, Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D, Nat. Mater., 2017, 16, 1272 CrossRef CAS PubMed.
- S. Goel, S. I. Zones and E. Iglesia, Encapsulation of metal clusters within MFI via interzeolite transformations and direct hydrothermal syntheses and catalytic consequences of their confinement, J. Am. Chem. Soc., 2014, 136, 15280–15290 CrossRef CAS PubMed.
- H. J. Cho, D. Kim, J. Li, D. Su and B. J. Xu, Zeolite-encapsulated Pt nanoparticles for tandem catalysis, J. Am. Chem. Soc., 2018, 140, 13514–13520 CrossRef CAS PubMed.
- W. Li, Y. H. Deng, Z. X. Wu, X. F. Qian, J. P. Yang, Y. Wang, D. Gu, F. Zhang, B. Tu and D. Y. Zhao, Hydrothermal etching assisted crystallization: A facile route to functional yolk-shell titanate microspheres with ultrathin nanosheets-assembled double shells, J. Am. Chem. Soc., 2011, 133, 15830–15833 CrossRef CAS PubMed.
- J. Du, J. Qi, D. Wang and Z. Y. Tang, Facile synthesis of Au@TiO2 core-shell hollow spheres for dye-sensitized solar cells with remarkably improved efficiency, Energy Environ. Sci., 2012, 5, 6914–6918 RSC.
- N. Zhang, S. Q. Liu, X. Z. Fu and Y. J. Xu, Synthesis of M@TiO2 (M = Au, Pd, Pt) core-shell nanoconnposites with tunable photoreactivity, J. Phys. Chem. C, 2011, 115, 9136–9145 CrossRef CAS.
- W. G. Tu, Y. Zhou, H. J. Li, P. Li and Z. G. Zou, Au@TiO2 yolk-shell hollow spheres for plasmon-induced photocatalytic reduction of CO2 to solar fuel via a local electromagnetic field, Nanoscale, 2015, 7, 14232–14236 RSC.
- P. Zhang and X. W. Lou, Design of heterostructured hollow photocatalysts for solar-to-chemical energy conversion, Adv. Mater., 2019, 31, 1900281 CrossRef PubMed.
- N. Wang, Q. M. Sun, R. S. Bai, X. Li, G. Q. Guo and J. H. Yu, In situ confinement of ultrasmall Pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation, J. Am. Chem. Soc., 2016, 138, 7484–7487 CrossRef CAS PubMed.
- J. B. Cai, X. Q. Wu, S. X. Li, F. Y. Zheng, L. C. Zhu and Z. H. Lai, Synergistic effect of double-shelled and sandwiched TiO2@Au@C hollow spheres with enhanced visible-light-driven photocatalytic activity, ACS Appl. Mater. Interfaces, 2015, 7, 3764–3772 CrossRef CAS PubMed.
- Z. F. Jiang, W. Wei, D. J. Mao, C. Chen, Y. F. Shi, X. M. Lv and J. M. Xie, Silver-loaded nitrogen-doped yolk-shell mesoporous TiO2 hollow microspheres with enhanced visible light photocatalytic activity, Nanoscale, 2015, 7, 784–797 RSC.
- M. G. Wang, J. Han, H. X. Xiong and R. Guo, Yolk@shell nanoarchitecture of Au@rGO/TiO2 hybrids as powerful visible light photocatalysts, Langmuir, 2015, 31, 6220–6228 CrossRef CAS PubMed.
- B. Cao, G. S. Li and H. X. Li, Hollow spherical RuO2@TiO2@Pt bifunctional photocatalyst for coupled H2 production and pollutant degradation, Appl. Catal., B, 2016, 194, 42–49 CrossRef CAS.
- Q. R. He, H. Sun, Y. X. Shang, Y. N. Tang, P. She, S. Zeng, K. L. Xu, G. L. Lu, S. Liang, S. Y. Yin and Z. N. Liu, Au@TiO2 yolk-shell nanostructures for enhanced performance in both photoelectric and photocatalytic solar conversion, Appl. Surf. Sci., 2018, 441, 458–465 CrossRef CAS.
- K. E. deKrafft, C. Wang and W. B. Lin, Metal–organic framework templated synthesis of Fe2O3/TiO2 nanocomposite for hydrogen production, Adv. Mater., 2012, 24, 2014–2018 CrossRef CAS PubMed.
- J. K. Zhang, Z. B. Yu, Z. Gao, H. B. Ge, S. C. Zhao, C. Q. Chen, S. Chen, X. L. Tong, M. H. Wang, Z. F. Zheng and Y. Qin, Porous TiO2 nanotubes with spatially separated platinum and CoOx cocatalysts produced by atomic layer deposition for photocatalytic hydrogen production, Angew. Chem., Int. Ed., 2017, 56, 816–820 CrossRef CAS PubMed.
- L. J. Gao, Y. G. Li, J. B. Ren, S. F. Wang, R. N. Wang, G. S. Fu and Y. Hu, Passivation of defect states in anatase TiO2 hollow spheres with Mg doping: realizing efficient photocatalytic overall water splitting, Appl. Catal., B, 2017, 202, 127–133 CrossRef CAS.
- J. Q. Yan, H. Wu, H. Chen, Y. X. Zhang, F. X. Zhang and S. F. Liu, Fabrication of TiO2/C3N4 heterostructure for enhanced photocatalytic Z-scheme overall water splitting, Appl. Catal., B, 2016, 191, 130–137 CrossRef CAS.
- X. Yu, J. Zhang, Z. H. Zhao, W. B. Guo, J. C. Qiu, X. N. Mou, A. X. Li, J. P. Claverie and H. Liu, NiO–TiO2 p–n heterostructured nanocables bridged by zero-bandgap rGO for highly efficient photocatalytic water splitting, Nano Energy, 2015, 16, 207–217 CrossRef CAS.
- P. F. Wang, S. H. Zhan, Y. G. Xia, S. L. Ma, Q. X. Zhou and Y. Li, The fundamental role and mechanism of reduced graphene oxide in rGO/Pt–TiO2 nanocomposite for high-performance photocatalytic water splitting, Appl. Catal., B, 2017, 207, 335–346 CrossRef CAS.
- K. F. Wu, H. M. Zhu, Z. Liu, W. Rodriguez-Cordoba and T. Q. Lian, Ultrafast charge separation and long-lived charge separated state in photocatalytic CdS–Pt nanorod heterostructures, J. Am. Chem. Soc., 2012, 134, 10337–10340 CrossRef CAS PubMed.
- K. F. Wu, H. M. Zhu and T. Q. Lian, Ultrafast exciton dynamics and light-driven H2 evolution in colloidal semiconductor nanorods and Pt-tipped nanorods, Acc. Chem. Res., 2015, 48, 851–859 CrossRef CAS PubMed.
- J. B. Lee, S. Choi, J. Kim and Y. S. Nam, Plasmonically-assisted nanoarchitectures for solar water splitting: obstacles and breakthroughs, Nano Today, 2017, 16, 61–81 CrossRef CAS.
- V. Subramanian, E. E. Wolf and P. V. Kamat, Catalysis with TiO2/gold nanocomposites. effect of metal particle size on the Fermi level equilibration, J. Am. Chem. Soc., 2004, 126, 4943–4950 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional characterization results including SEM images, TEM images, EDS mapping images, XRD patterns, and time courses of photocatalytic H2 evolution. See DOI: 10.1039/d0qm00042f |
| This journal is © the Partner Organisations 2020 |





