Eighteen questions and answers about the effects of the depletion of the ozone layer on humans and the environment
Pieter J. Aucamp (coordinator)
School for Environmental Science and Management, Potchefstroom University for CHE, PO Box 915751, Faerie Glen, 0043, South Africa. E-mail: pjaucamp@iafrica.com
First published on 10th January 2003
Introduction
The Meetings of the Parties to the Montreal Protocol appointed three Assessment Panels to review the progress in scientific knowledge on their behalf. These panels are: the Scientific Assessment Panel, the Technological and Economic Assessment Panel and the Environmental Effects Assessment Panel. Each panel covers a designated area and there is very little overlap between their reports. The main reports are published every four years as required by the Meetings of the Parties. All these reports have an executive summary that is distributed more widely than the main report itself. It became customary to add a set of questions and answers – mainly for lay readers – to the report. This document contains the questions and answers prepared by members of the Environmental Assessment Panel. It is based mainly on the 2002 report of the Effects Panel but also contains information from previous assessments. Readers who need more detailed information on any of the questions are referred to these full reports for a more complete scientific discussion.This set of questions refers mainly to the environmental effects of ozone depletion. The report of the Scientific Assessment Panel contains questions and answers related to the other scientific issues addressed by that Panel. All the reports can be found on the UNEP website (http://www.unep.org/ozone).
Q1 What is ozone and how do we define ultraviolet radiation?
Ozone is a gas that occurs in the atmosphere. It is formed in the stratosphere by the action of ultraviolet radiation from the sun on oxygen. The ozone molecule contains three atoms of oxygen. It is also formed near the Earth's surface in chemical reactions caused by man-made pollution. Ozone absorbs a part of the ultraviolet radiation from the sun, thereby reducing the potentially dangerous UV-B radiation reaching the surface of the earth.Ozone is a strong oxidising agent. It will destroy any organic material on contact by oxidising the molecules of the cells. This property is often used to sterilise drinking and swimming pool water. Inhaling ozone can be harmful to mammals in small doses and toxic in large doses. In the lower atmosphere ozone is one of the detrimental components of photochemical smog that is produced as the result of pollution (Bad ozone). However, in the stratosphere, ozone absorbs ultraviolet radiation and protects the environment against the detrimental effects of the UV-B radiation (Good ozone).
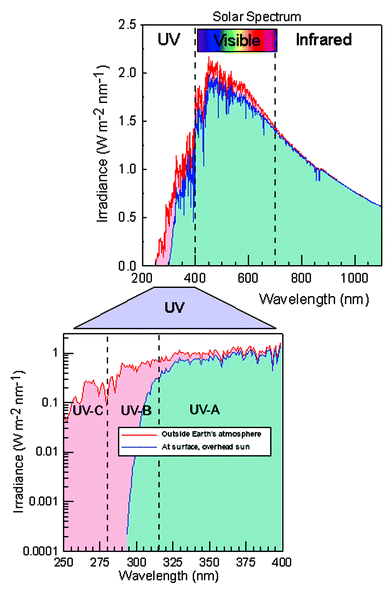
The radiation emitted by the sun contains an ultraviolet component. This covers the range with wavelengths from 100 to 400 nm and is divided into three bands: UV-A (315–400 nm), UV-B (280–315 nm) and UV-C (100–280 nm). As the sunlight passes through the atmosphere, no UV-C and approximately 10% of the UV-B is transmitted. They are absorbed mainly by ozone and oxygen. UV-A radiation is less affected by the atmosphere. Therefore, the ultraviolet radiation reaching the Earth's surface is composed of mainly UV-A with a small UV-B component. A decrease in the concentration of ozone in the atmosphere results in increased UV-B levels. DNA and other biological macromolecules absorb UV-B and can be damaged in the process.
Q2 What determines the level of UV-B at a particular location?
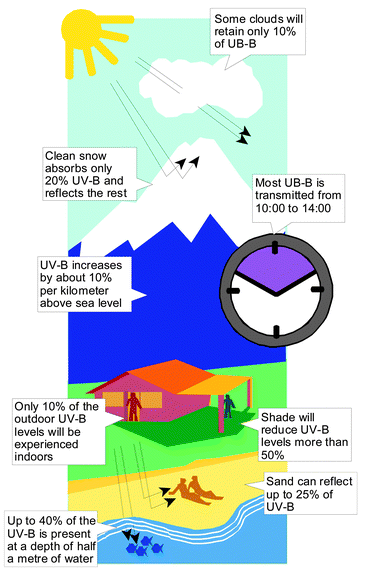
The sun is the origin of ultraviolet radiation reaching the earth. This radiation is absorbed by the components of the Earth's atmosphere including ozone. At higher latitudes the rays of the sun have to take a longer path through the atmosphere before they reach the Earth. In general, the level of ultraviolet radiation is higher at the equator and diminishes towards the poles. The levels increase with elevation above sea level. Clouds, particulate matter and aerosols absorb ultraviolet radiation and diminish ultraviolet levels. The level of the ultraviolet radiation at any particular location is determined by a combination of all these factors.The UV-B levels vary with the time of day and season. The highest level occurs when the sun is at its maximum elevation, around midday during the summer months. The radiation levels are higher in the intertropical regions.
On average, the highest ultraviolet radiation levels occur on cloudless days. Cloud cover reduces the radiation but it can still be high under partly cloudy conditions when the sun is not obscured. Evidence shows that over the time span of satellite-based ozone measurements, changes in cloud cover have been much less important than stratospheric ozone reductions in causing surface UV changes.
Higher elevations have less atmosphere overhead, as evidenced by the thinner air and lower atmospheric pressure. The increase in UV radiation varies between 10% and 20% for each kilometre of elevation, the exact number depending on the specific wavelength, solar angle, reflections, and other local conditions.
Frequently, other factors besides thickness of the atmosphere cause even larger differences in UV radiation between elevations. Snow is more common at higher elevations, and reflects as much as 90% of the ultraviolet radiation. Dry beach sand reflects about 15% and sea foam about 25%.
Q3 What is the effect of atmospheric pollution on the level of UV-B?
Man-made pollution often contains oxides of nitrogen and sulfur and various hydrocarbons. Chemical reactions between these compounds can form ozone. This mixture of gaseous compounds and particulate matter appears as a brown haze known as photochemical smog. The ozone in the smog will also absorb ultraviolet radiation.While most of the atmospheric ozone resides in the stratosphere, some ozone is made in the troposphere by the chemical reactions of pollutants such as nitrogen oxides and hydrocarbons. This tropospheric ozone is a component of the photochemical smog found in many polluted areas. Airborne particles (smoke, dust, sulfate aerosols) block UV radiation, but at the same time can increase the amount of scattered light (haze) and therefore increase the UV exposure of side-facing surfaces (e.g., face, eyes). Comparisons of measurements made in industrialised regions of the Northern Hemisphere (e.g. central Europe) and in very clean locations at similar latitudes in the Southern Hemisphere (e.g., New Zealand) indicate the importance of particulate and pollution-related UV-B reductions. At any particular location there is a direct relationship between UV-B levels and the amount of ozone in the atmosphere. UV-B increases with ozone depletion in the stratosphere but decreases with ozone formation in the lower atmosphere. The natural UV-B variability (e.g., from time of day, or clouds) can be larger, but goes in both directions, up and down. Many detrimental effects of UV-B are proportional to the cumulative UV-B exposure.
Q4 What is the solar UV index?
The solar UV index (UVI) describes the level of solar UV radiation at the Earth's surface. The values of the index range from zero upward – the higher the index value, the greater the potential for damage to the skin and eye, and the less time it takes for harm to occur. The UV index is computed using forecasted ozone levels, a computer model that relates ozone levels to UV incidence on the ground, forecasted cloud amounts, and the elevation of the forecast cities. Some countries also use ground observations.The calculation starts with measurements of current total ozone amounts. The data are then used to produce a forecast of ozone levels for the next day at various points. A model is used to determine the amount of UV radiation with wavelengths from 290 to 400 nm reaching the ground. The time of day (solar noon), day of year, and latitude are used. The information is then weighted according to how human skin responds to each wavelength. It is more important to protect people from wavelengths that harm skin than from wavelengths that do not damage the skin.
The weighted irradiances are integrated over the 290 to 400 nm range resulting in a value representing the effect a given UV radiation intensity will have on skin. The estimates are then adjusted for the effects of elevation and clouds. UV at the surface increases by about 10% per kilometre above sea level. Clear skies allow 100% of the incoming UV radiation from the sun to reach the surface, whereas scattered clouds transmit about 90%, broken clouds about 75%, and overcast conditions about 30%. Once adjusted for elevation and clouds, this value is then scaled by a conversion factor. This results in a number that usually ranges from 0 (where there is no sunlight) to the mid teens. This value is called the UV index. Ideally, the computation of the UV index should include the effects of variable surface reflection (e.g., sand, water, or snow), atmospheric pollutants or haze.
| Exposure category | UVI range |
|---|---|
| Low | < 2 |
| Moderate | 3–5 |
| High | 6–7 |
| Very High | 8–10 |
| Extreme | 11+ |
Q5 How does the UV index vary with geographical location and season?
The combination of total ozone and solar zenith angle, which is determined by the geographical position, season and time of the day, can lead to a variety of situations.Modelled noon UV index calculated under clear sky conditions at 20°, 30° and 60°S is shown in the figure. The total ozone column values selected for calculation at 20° and 30° are typical values observed at those latitudes, while the ozone column value used for calculations at 60° are observed under ozone hole conditions. Values around 150 Dobson Units (DU) can be present at 60°S during October, while 200 DU have been observed later in the spring.
The UV index at 60°S under a total ozone column of 200 DU is always lower than the value present at 30°S. On the other hand, the UV index calculated for 150 DU would only exceed those at 30°S if that ozone total column is present at the end of October or later, and exceed those at 20° if present later than the middle of December.
The presence of “patchy” skies or snow-covered ground can result in irradiances larger than those shown in the figure. Altitude can increase the UV index. A good example is the intertropical high altitude desert of Puna de Atacama in Argentina where a UV index of 18 is common in January and December, with a maximum of 20 and even more on certain days. A combination of low solar zenith angle near noon, high altitude, a naturally low ozone total column and very clean atmosphere is responsible for these exceptionally high values.
Q6 What is the effect of exposure to UV-B on mammals?
The cells of three different systems can be directly exposed to UV radiation—the eyes, the skin, and the immune system. Acute exposure of the eyes to UV radiation causes photokeratitis (snow blindness) and chronic exposure contributes to cataract formation. In the skin, UV irradiation causes sunburn, photoaging, and skin cancer.Moderate exposure to sunlight in the course of everyday life is essential for health. UV-B radiation is involved in the formation of vitamin D, which is necessary for growth and maintenance of bones and teeth. There is some evidence suggesting that maintaining normal vitamin D levels protects against the development of colon, breast, and prostate cancers. However, very moderate exposure is all that is required, and excessive exposure confers no added benefit.
Skin cancer is found in almost all animals that have been studied in the long-term, for example, cattle, goats, sheep, cats, dogs, guinea pigs, rats, and mice. Direct effects of UV-B radiation on body parts that are covered by thick hair are negligible. However, even furred animals usually have exposed skin around the mouth, nostrils and on other parts of the body. These parts, unless they are heavily pigmented, can be damaged by radiation.
Q7 What is the effect of exposure to UV-B on the skin?
Acute exposure of the skin to UV radiation causes sunburn. The amount of UV required to produce sunburn depends on the absorption in the superficial layers (i.e. the thickness and amount of pigment) of the skin and on other genetic factors. The efficiency with which sunlight produces sunburn depends on the amount of UV-B radiation it contains. For example, more UV-B is present at high altitudes and more is present in noontime sun than at earlier or later hours. Chronic exposure of the skin to UV radiation also causes wrinkling, thinning, and loss of elasticity of the skin (photoaging); however, UV-A radiation may be more important than UV-B radiation in causing these changes.Sunscreens are designed to protect against sunburn and can be highly effective in this regard. There is also evidence that they reduce the incidence of squamous cell carcinoma and precancerous lesions in the skin. Sunscreens can also provide protection against the photoaging and immunological effects of UV radiation, particularly if they contain chemicals that absorb both UV-B and UV-A radiation. There is no evidence that getting a suntan will help prevent skin cancer. The UV exposure needed to acquire the tan adds to the skin cancer risk. The fact that one is able to tan well does, however, signify that the personal risk is lower (by a factor of 2 to 3) than for people who do not tan. Naturally dark-skinned people have a built-in protection of their skin against sunlight.
Basal and squamous cell carcinomas occur most often and with high frequency in light-skinned Caucasians living in sunny climates. Fortunately, most of these skin cancers are readily treated and are rarely fatal. Cutaneous melanoma is much more dangerous, but occurs with a much lower frequency than the other types. Its relationship to UV radiation is not well understood, but exposure early in life seems to be an important factor in the subsequent development of melanoma. Light-skinned populations have the highest risk of developing melanoma. Although melanoma can occur in highly pigmented persons, such cancers are often not related to sun exposure.
UV irradiation causes skin cancers by altering critical genes that control cell division and cell death. Altered genes result from the ability of UV to make chemical alterations in DNA, the building block of genes. Some of the genes involved in skin cancer development have been identified. These include the p53 tumour suppressor gene (squamous and basal cell carcinomas), the PTCH gene (basal cell carcinomas), the p16 gene (melanomas), and a variety of genes involved in the repair of UV-damaged DNA (all types).
Q8 How does UV-B affect the immune system?
The immune system can be altered by UV irradiation, leading to diminished immune response to infectious agents and some cancers.Some cells of the immune system, called antigen-presenting cells, reside in the skin. Their function is to capture invading micro organisms and carry them to the lymph nodes, where the immune response is initiated. These cells can be damaged directly by UV radiation and will then no longer be able to initiate an immune response or produce an aberrant one. Other cells in the skin produce chemical mediators that direct the immune response toward either immune suppression or active immunity. Exposing these cells directly to UV-B radiation stimulates the release of mediators that favour the development of immune suppression. Finally, a molecule in the keratin layer of the skin, urocanic acid, undergoes a chemical change in response to UV radiation, which allows it to trigger the release of chemical mediators from mast cells in the skin, which also divert the immune response toward a pathway of immune suppression.
What is the significance of UV-induced immune suppression for human diseases? The answer is not yet clear. Although there are some examples in which UV exposure increases susceptibility to and the severity of an infection, such as with herpes virus infections (cold sores, shingles), the full implications of the immunological effects are unknown. Numerous laboratory animal models of infectious diseases demonstrate that exposure to UV radiation at a critical time during infection can increase the severity and duration of the disease. Also, UV exposure during immunisation can reduce the effectiveness of vaccinations. How these observations may apply to human diseases remains a subject of intense interest and research.
Q9 What are the effects of increased UV-B radiation on crops and forests?
Most plants have UV shielding, but not always sufficient for complete protection. Only a small proportion of the UV-B radiation striking a leaf penetrates into the inner tissues. When exposed to enhanced UV-B radiation, many species of plants can increase the UV-absorbing pigments in their outer leaf tissues. Other adaptations may include increased thickness of leaves that reduces the proportion of inner tissues exposed to UV-B radiation and changes in the protecting waxy layer of the leaves. Several repair mechanisms exist in plants, including repair systems for DNA damage or oxidant injury. The net damage a plant experiences is the result of the balance between damage and protection and repair processes.There are some UV-B-sensitive varieties of crops that experience reductions in yield. There are also UV-B-tolerant varieties, providing the opportunity to breed and genetically engineer for UV-B tolerant crops. For commercial forests, tree breeding and genetic engineering may be used to improve UV-B tolerance. While many forest tree species appear to be UV-B tolerant, there is limited evidence that detrimental UV-B effects accumulate slowly from year to year in sensitive species. The biochemistry and physiology of plants are influenced by UV-B exposure such as in the accumulation of UV-B pigments. It is not possible to conclude whether or not the changes will have any appreciable impact on the quality of food.
Plants and animals have, during their evolution, adapted to particular environments. They have acquired protection and repair mechanisms appropriate for their particular situations. However, the present rate of global change is so rapid that evolution may not keep up with it, particularly in long-lived plants like trees. Thus, plants adapted to low UV-B environments may suffer even from an increase that is smaller than the difference between natural levels at the equator and higher latitudes. For example, herbaceous plants native to the southern tip of South America (Tierra del Fuego, Argentina) and the Antarctic Peninsula have been shown to be affected by the current ambient UV-B levels. Over long times and many generations, there is the possibility that genetic adaptation can develop.
Q10 What is the effect of UV-B exposure on aquatic life?
Pure water is almost transparent to UV radiation. A beam of UV-B radiation can penetrate more than 500 metres through pure water before it is completely absorbed. Natural waters contain UV-absorbing substances, such as dissolved organic matter, that partly shields aquatic organisms from UV-B, although the degree of shielding varies widely from one water body to another.In clear ocean and lake waters ecologically significant levels of UV-B can penetrate to tens of metres. In turbid rivers and wetlands, however, UV-B may be completely absorbed within the top few centimetres. Most organisms in aquatic ecosystems, such as phytoplankton, live in the illuminated euphotic zone close to the water surface where exposure to UV-B can occur. In particular, UV-B radiation may damage those organisms that live at the surface of the water during their early life stages.
Most adult fish are well protected from excessive solar UV, since they inhabit deep waters. Some shallow water fish have been found to develop skin cancer and other UV-related diseases. The eggs and larvae of many fish are sensitive to UV-B exposure. In the Gulf of Maine, UV penetrates to considerable depth where the embryos and larvae of the Atlantic cod develop. Exposure to UV equivalent to 10 m depth resulted in a significant mortality of developing embryos and a significant decrease in length of larvae. These irradiances occur in many temperate latitudes where these ecologically and commercially important fish spawn. In contrast, lobster larvae seem to be tolerant to UV radiation even though they develop in the surface layers of the water column.
Q11 Does global warming alter the effect of UV radiation on aquatic ecosystems?
Climate change may result in temperature and sea level changes, shifts in the timing and extent of sea ice cover, changes in wave climate, ocean circulation, salinity and altered stratification of the water column. These complex changes are likely to have significant impacts that will vary both spatially and temporally. These changes will affect biological systems (including human marine resources), the global hydrological cycle, vertical mixing and efficiency of carbon dioxide uptake by the ocean. Ozone-related increases in UV-B are an important additional ecological stress that will have both positive and negative impacts in association with the other factors.Increasing carbon dioxide concentrations will result in warming of the troposphere and simultaneous cooling of the stratosphere, which favours further ozone destruction. One of the possible feedback mechanisms is change in cloud cover and increased rainfall, but this is not well understood. Global warming changes the amount of ice and snow cover in polar and sub-polar areas. Ice and snow strongly attenuate the penetration of solar radiation into the water column. Therefore, any substantial decrease in ice and snow cover will alter the exposure of aquatic ecosystems to solar UV radiation. Another aspect is the dependence of many physiological responses on temperature. Enzymatic repair mechanisms are inhibited by low temperature, while elevated temperatures may augment enzymatic repair mechanisms.
Q12 What are the effects of stratospheric ozone depletion on certain processes and cycles in the environment?
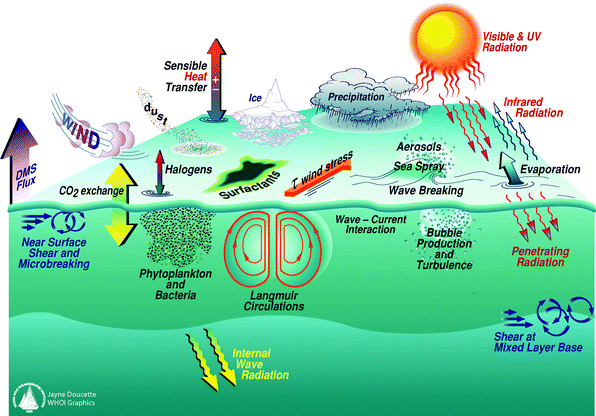
Ozone depletion results in greater amounts of UV-B radiation that will have an impact on terrestrial and aquatic biogeochemical systems. Biogeochemical cycles are the complex interactions of physical, chemical, geological and biological processes that control the transport and transformation of substances in the natural environment and therefore the conditions that humans experience in the Earth's system. The increased UV-B radiation impinging on terrestrial and aquatic systems, due to ozone depletion, results in changes in the trace gas exchange between the continents, oceans and the atmosphere. This results in complex alterations to atmospheric chemistry, the global elemental cycles, such as the carbon cycle, and may have an impact on the survival and health of all organisms on Earth, including humans.In the figure, UV radiation is shown to influence oceanic primary productivity and the production of trace gases at the surface of the oceans and subsequent transfer to the atmosphere. Once in the atmosphere, trace gases such as CO2 interact with the physical climate system resulting in alterations to climate and feedbacks in the global biogeochemical system. Since atmospheric CO2 concentrations play a central role in determining the distribution of heat in the atmosphere, the multiple complex components of the physical climate system such as wind, air–sea momentum, heat exchange and precipitation are influenced. There are also similarly complex interactions between biogeochemical cycling on land and the integrated climate system that have important implications for organisms on Earth. At this stage it is not possible to predict the overall effects of these complex interactions.
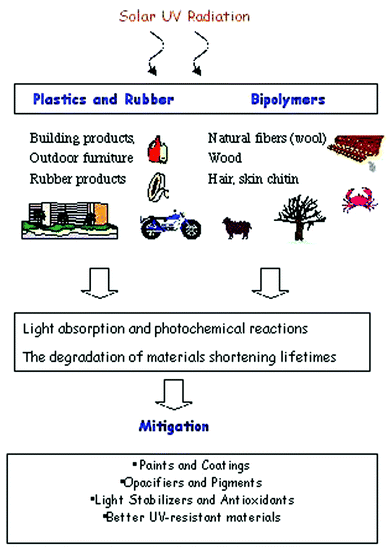
Q13 Do increased UV-B levels shorten the lifetime of commercial polymers?
The outdoor service life of commercial plastics is often limited by their UV-stability and weatherability. The outdoor lifetimes of plastics, however, depend on their formulations and specifically on the type and concentration of photostabiliser additives used in them. Information on the lifetimes of unstabilised (virgin) plastic materials is therefore of limited practical value in assessing the UV stability of plastics exposed outdoors.Available data on the degradation of plastics by UV in sunlight show that for some polymers a portion of the damage is attributable to the UV-B radiation component. As any depletion of the stratospheric ozone layer would increase the UV-B content of the solar radiation reaching the Earth's surface, it is reasonable to expect a consequent increase in the rate of degradation of plastics containing these polymers (and other materials such as wood) under these conditions. The amount by which the service life of materials will be shortened by this phenomenon will of course depend on the type of polymer, location in question, and the light-stabiliser used in the formulation.
Climatic factors, particularly higher ambient temperatures due to global warming, will tend to accelerate UV-induced degradation to an extent depending on the type of plastic and on the location of exposure.
These reductions in service life of plastics can probably be countered by using higher than normal concentrations of existing light stabilisers in the plastic formulation, surface protection of materials (e.g. painting wood) or by selecting different polymeric materials with better UV-resistant materials for outdoor applications. These approaches might be able to retain the service life at present-day levels but may increase the cost of relevant products.
Q14 Will stratospheric ozone depletion change air quality, and how does this relate to global warming?
Stratospheric ozone depletion normally increases the ozone concentration at ground level. In general the impact of stratospheric ozone depletion is smaller than that of local and regional air pollution sources. Increases in the particulates in the atmosphere related to global warming may reduce tropospheric ozone production. Modelling and field studies show that the reduction of ozone photolysis rate and ozone production in the troposphere are expected in the presence of increased amounts of absorbing aerosols in the boundary layer.Climate change can alter air quality in many ways. Changes in temperature, winds and cloudiness can all be important. Some of these changes will also alter the impact of stratospheric ozone depletion.
As an example, an increase in atmospheric CO2 concentration would accelerate photosynthesis, which might enhance the emissions of biological volatile organic compounds from forests and other natural ecological systems. Other sources of tropospheric air pollutants may be affected by global warming. It is known that local and large-scale biomass fires, such as are used for land-clearing, are rich sources of nitrogen oxides, carbon monoxide, methane, and other non-methane hydrocarbons, that can lead to enhanced tropospheric ozone production. Climate changes resulting from global warming may increase the risk of large-scale forest and brush fires and so affect concentrations of tropospheric air pollutants. The resulting particulates in the atmosphere can scatter sunlight, thus improving the efficiency of UV-B absorption of the boundary layer ozone and contributing to global warming.
Q15 What risks do the breakdown products of HFCs and HCFCs present to humans and the environment?
The new hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs) replacements for the chlorofluorocarbons (CFCs) are largely degraded before reaching the stratosphere. The final breakdown products are various fluorinated and chlorinated acetic acids. Some of these are rapidly broken down by microbiological activity in water, soil, and sediments. Other breakdown products such as trifluoroacetic acid (TFA) are very persistent but they are very water soluble and chemically non-reactive. Because of their properties, these breakdown products will ultimately collect in aquatic environments. They have low toxicity to aquatic organisms and are very unlikely to adversely affect human health or the environment.HFCs and HCFCs (CF3CXYH) break down relatively rapidly to several products including the persistent substances such as trifluoroacetic acid (CF3COOH) and chlorodifluoroacetic acid (CF2ClCOOH). These are washed from the atmosphere by precipitation and reach surface waters, along with other chemicals washed from the soil. In locations where there is little or no outflow and high evaporation (seasonal wetlands, salt lakes and playas), these products are expected to increase in concentration over time. The concentrations of trifluoroacetic acid and chlorodifluoroacetic acid are expected to increase. While this may present a risk to aquatic organisms, these areas would also experience increases in concentrations of other water-soluble materials such as has already occurred. The effects of increased concentrations of these naturally occurring salts and other materials would likely be greater and more biologically significant than those of breakdown products of the HFCs and HCFCs.
The results of this interaction with global climate change are judged to be of low significance, since the phytotoxicity of trifluoroacetic acid is not high.
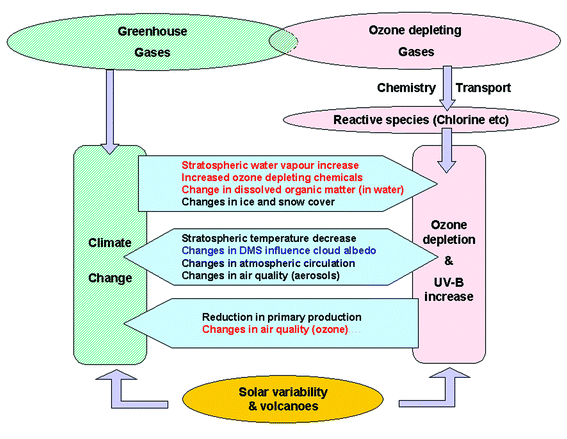
Q16 Is ozone depletion affected by global warming?
The interactions are complex, and not all of them are fully understood (see schematic). In the past, scientists have sometimes stressed the differences between global warming and ozone depletion. Global warming is due to a build-up of gases that absorb outgoing infrared radiation, especially CO2, while ozone depletion is primarily due to a release of gases that catalytically destroy ozone.Ozone, the CFCs and their substitutes are minor greenhouse gases. Several other gases involved with the chemistry of ozone depletion are also greenhouse active. These include water vapour, methane (CH4), and nitrous oxide (N2O) that are increasing and will ultimately lead to increases in stratospheric gases that catalytically destroy ozone. There are several radiative feedback processes involved. Increases in temperature can lead to changes in cloud cover, rainfall patterns, ice accumulation, surface albedo, and ocean circulation. The direct radiative forcing from increases in UV-B that results from changes in ozone are not significant, since only a small fraction of the incoming solar energy falls within the UV-B range. However, increases in UV radiation at the Earth's surface influence photochemical reactions in the troposphere that would affect the lifetimes of greenhouse gases. It has been suggested that changes in cloud cover can be induced by climate change. Changes in solar output and future volcanic eruptions will influence both global warming and ozone depletion.
It seems that while current ozone depletion is dominated by chlorine and bromine in the stratosphere, in the longer term (∼100 years) the impact of climate change will dominate, through the effects of changes in atmospheric dynamics and chemistry. The result is that over the first half of the current century, increases in greenhouse gases may contribute to cooler stratospheric temperatures, since they act as infrared emitters in the stratosphere. This will lead to a decrease in the rate of catalytic destruction of ozone outside Polar regions. In Polar regions, however, the cooler temperatures may lead to increased polar stratospheric clouds, thus exacerbating ozone depletion. The temperature changes will lead to changes in atmospheric circulation. These changes may aid the mixing of long-lived CFCs from the troposphere to the stratosphere, which will increase their rate of photochemical destruction, again contributing to a faster recovery of ozone. Changes in polar ozone can also lead to changes in tropospheric circulation patterns, which in turn affect surface climate.
The effects of global warming on UV radiation are twofold. The first effect results from changes in global warming that influence total ozone. The second effect results from climate change effects on other variables such as clouds, aerosols, and snow cover that influence UV directly.
Q17 In what way is the Montreal Protocol beneficial?
Several attempts have been made to investigate the economic impacts of the problem of a depleted ozone layer. Such attempts meet with many problems. There are good reasons for concern for effects on humans, animals, plants and materials, but most of these cannot be estimated in monetary terms. Calculating the overall economic impact of such effects is difficult. Economic terms apply only to some of the effects, such as the cost of medical treatments, and the loss of production in fisheries and agriculture, and damage to materials; but how does one calculate the cost equivalent to the suffering of a blind or dying person, or the loss of a rare plant or animal species?The most comprehensive study was initiated by Environment Canada in 1997 for the 10th anniversary of the Montreal Protocol on Substances that Deplete the Ozone Layer. In this study the costs were calculated for all measures taken internationally to protect the ozone layer, such as replacement of technologies using ozone-depleting substances. The benefits are the total value of the damaging effects avoided in this way. The total costs of the measures taken to protect the ozone layer were calculated to be 235 billion US dollars.
Q18 Where can I get more information about the science and effects of ozone depletion?
There are many websites that contain information on ozone, UV, environmental effects and related topics. However, some are outdated and some may contain incorrect information. The sites mentioned below belong to dependable organisations and contain dependable information. Most of these sites contain links to other sources of information.| UNEP | www.unep.org |
| WMO | www.wmo.ch |
| WHO | www.who.int |
| IPCC | www.ipcc.ch |
| NOAA | www.al.noaa.gov |
| EPA | www.epa.gov |
| NASA | http://jwocky.gsfc.nasa.gov |
| NIWA | www.niwa.co.nz |
| Environment Canada | www.ec.gc.ca |
| This journal is © The Royal Society of Chemistry and Owner Societies 2003 |