Ganesh Pandey, Manmohan Kapur, M. Islam Khan and Sushama M. Gaikwad
Org. Biomol. Chem., 2003,1, 3321-3326
DOI:
10.1039/B307455B,
Paper
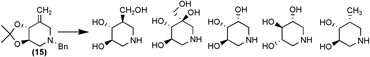
A new synthetic strategy has been devised to access a variety of polyhydroxylated