M. Agostinha R. Matos, Margarida S. Miranda, Victor M. F. Morais and Joel F. Liebman
Org. Biomol. Chem., 2003,1, 2566-2571
DOI:
10.1039/B304405J,
Paper
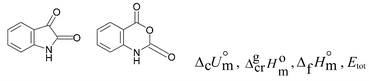
This paper reports the results of our thermochemical/calorimetric determination of the enthalpies of combustion, phase change, and formation of isatin, isatoic anhydride, and N-methylisatin. The density functional calculations accompanied by vibrational and thermal corrections were also performed for these compounds and N-methylisatoic anhydride. Through a combination of theoretical calculations and associated isodesmic reactions, we have deduced that isatin has some antiaromatic character and isatoic anhydride enjoys some aromatic stabilization.