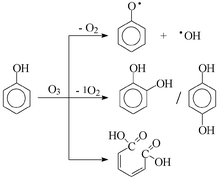
In the ozonolysis of phenol in aqueous solution at pH 3, 7 and 10 the following products were quantified: catechol, hydroquinone, 1,4-benzoquinone, cis,cis-muconic acid, H2O2, 2,4-dihydroxybiphenyl and 4,4-dihydroxybiphenyl. At pH 10, material balance (products vs. phenol consumption) is obtained. Singlet dioxygen, O2(1Δg), and ˙OH are formed as short-lived intermediates. The precursor of the latter, O3˙−, and a phenoxyl radical is suggested to arise from electron transfer from phenol/phenolate to ozone. Addition of ˙OH to phenol gives rise to dihydroxycyclohexadienyl radicals which add dioxygen and eliminate HO2˙ thereby forming catechol/hydroquinone. In competition and catalysed by H+ and OH−, the dihydroxycyclohexadienyl radical eliminates water yielding a phenoxyl radical. At pH 10, they readily oxidise catechol and hydroquinone. This reforms phenol (accounting for the low phenol consumption) and yields higher-oxidised products, eventually 1,4-benzoquinone. cis,cis-Muconic acid can be accounted for by the Criegee mechanism, while O2(1Δg) is released on the way to (some of the) catechol and hydroquinone.
Similar reactions proceed with hydroquinone (products: 1,4-benzoquinone, 2-hydroxy-1,4-benzoquinone and H2O2, with high yields of O2(1Δg) and ˙OH) and with catechol (products: 2-hydroxy-1,4-benzoquinone, cis,cis-muconic acid, H2O2 with high yields of O2(1Δg) and ˙OH). Material balance is not obtained for these two systems.
Pentachlorophenolate, pentabromophenolate and 2,4,6-triiodophenolate ions give rise to halide ions, O2(1Δg)
(58%/48%/10%) and ˙OH (27%/2%/0%). It is suggested that together with O2(1Δg) the corresponding ortho- and para-quinones plus a halide ion are formed. Further halide ion is released upon the hydrolysis of these and other products. For pentachlorophenolate the material balance with respect to the short-lived intermediates is 85%. With the bromo- and iodophenolates the O2(1Δg) yields are substantially lowered, most likely due to release of triplet (ground state) dioxygen induced by the heavy atom effect.