Out of the oil bath and into the oven—microwave-assisted combinatorial chemistry heats up
Helen E. Blackwell*
Department of Chemistry, University of Wisconsin – Madison, 1101 University Ave., Madison, WI 53706-1396, USA. E-mail: blackwell@chem.wisc.edu
First published on 26th March 2003
Abstract
The application of microwave irradiation to expedite solid-phase organic reactions could be the tool that allows combinatorial chemistry to deliver on its promise—providing rapid access to large collections of diverse small molecules. Herein, several different approaches to microwave (MW)-assisted solid-phase reactions and library synthesis are introduced, including the use of solid-supported reagents, multicomponent coupling reactions, solvent-free parallel library synthesis, and spatially addressable library synthesis on planar solid supports. The future impact of MW-assisted organic reactions on solid-phase and combinatorial chemistry could prove to be immense, and methods for further improvement of this strategic combination of technologies are highlighted.
 Helen E. Blackwell Helen E. Blackwell | Helen Blackwell was born in Cleveland, Ohio and attended Oberlin College for her undergraduate studies, graduating with highest honors in chemistry in 1994. She then left the Midwest and went to the West coast to pursue her graduate studies in organic chemistry at the California Institute of Technology as an NSF predoctoral fellow with Robert Grubbs. Helen received her PhD in 1999 and then spent three years as Jane Coffin Childs postdoctoral fellow in the lab of Stuart Schreiber at Harvard University. In 2002, she returned to the Midwest and joined the faculty of the University of Wisconsin – Madison, where she is presently assistant professor of chemistry. |
Introduction
Instead of warming up your cup of coffee in the microwave while waiting for a solid-phase reaction to go to completion, why not put the reaction in the microwave in its place? This idea is an answer to a question perhaps many chemists have asked while waiting for a solid-phase organic reaction to proceed: “How could I speed this up?” In fact, many researchers are now investigating the acceleration of solid-phase reaction rates by performing them under microwave (MW) irradiation, and MW-assisted synthesis is emerging as a powerful approach to dramatically accelerate the pace of combinatorial chemistry. With new therapeutic targets emerging from genomic and proteomic research efforts, there is an urgent need to develop methods to synthesize small molecule modulators efficiently—MW-assisted combinatorial chemistry is poised to help chemists meet this challenge.This article outlines my own perspective on this exciting and fertile research area and is not meant as a comprehensive review.1 Rather, I give a background on solid-phase combinatorial chemistry and MW-assisted synthesis, and then present four areas of solid-phase combinatorial chemistry I believe have seen and will see the most impact from MW-assisted organic reactions. In particular, I will focus only on reaction schemes where one of the reagents is covalently attached to or supported on an insoluble polymeric (or inorganic) support (Fig. 1).
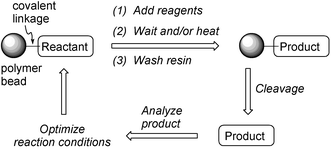 | ||
| Fig. 1 The solid-phase synthesis process on insoluble polymer beads. | ||
Solid-phase combinatorial chemistry
The synthesis of collections of different molecules by varying the combinations of molecular building blocks in each synthetic step is the core of the combinatorial chemistry strategy. This approach, which was in part enabled by the advent of solid-phase organic chemistry,2 has exploded within the chemistry community over the past decade, with applications ranging from drug discovery to catalyst design to materials science.3 The compound collections, commonly termed libraries, are prepared either in parallel or via a split-pool approach on solid-support.4 In either technique, selected sets of chemical building blocks are combined in a series of chemical reactions to give (theoretically) every small molecule outcome. The split-pool approach allows for significantly larger libraries to be prepared with less synthetic manipulation than parallel reactions, but requires extra post-synthesis deconvolution/decoding steps to determine the structure of each library member.5The purification benefits of having a target molecule covalently bound to insoluble polymeric supports and the ability to push reactions to completion using excesses of reagents are both features that make solid-phase organic synthesis an attractive platform for numerous combinatorial applications (Fig. 1). However, solid-phase reactions are heterogeneous and often take considerably longer than their homogeneous solution-phase counterparts (frequently up to 10 times). Reagent diffusion into the polymer matrix is invariably a slow process, especially for large macrobeads.6 As a result, solid-phase reactions often require more “forcing” reaction conditions than their solution-phase counterparts, such as high heat and prolonged reaction times—both conditions that can generate unwanted reaction by-products. Moreover, solid-phase reactions require extra steps to attach and detach the compound to and from the resin, and suffer from the lack of any qualitative method to quickly monitor their progress (e.g. thin layer chromatography, TLC). These drawbacks have become a bottleneck for solid-phase synthesis. I believe the inefficiency of solid-phase reactions has played a significant role in limiting the bounty many anticipated combinatorial chemistry would deliver.
MW-assisted synthesis—a primer
For combinatorial chemistry to deliver on its promise, general methods need to be discovered to accelerate solid-phase reactions to the point that they are equivalent in rate to, if not faster than, homogeneous reactions. Research groups around the globe have made this connection and have looked to the expanding field of MW-assisted organic synthesis to address this shortcoming (sometimes termed MW-enhanced or MW-accelerated chemistry). Over the past 15 years, the rates of an impressive number of organic transformations have been accelerated by subjecting them to MW irradiation.7 The majority of these have been homogeneous reactions, and in most cases reaction times of hours to days have been reduced to seconds to minutes. In principle, any reaction that requires heating for it to proceed at an appreciable rate should benefit from MW irradiation.Why does MW irradiation speed up organic reactions? This topic has been “hotly” debated, but the consensus in the recent literature appears to be that it is predominantly a thermal effect.8 Heating caused by MW irradiation is a result of dipole rotation and ionic conductance.9 MWs are a form of electromagnetic radiation, and most domestic and commercial MW ovens operate at a frequency of 2450 MHz. Molecules with a permanent dipole (such as the water in your coffee) that are subjected to this oscillating electric field will try to align themselves with the field. As the field oscillates at 4.9 × 109 times/second, these molecules are continuously aligning and realigning with the field. This rapid motion and resulting intermolecular friction cause an intense internal heat that can increase at rates up to 10 °C per second. This rapid heating, or “flash” heating, is most often cited to be the reason behind the dramatically accelerated reaction rates using MW irradiation. In support of this mechanism, the larger the relative permittivity of a substance, the greater the observed coupling with MWs.10
In contrast to the traditional, external heating of reaction vessels (e.g. in an oil bath or with a heating mantle), MW heating is a more homogeneous heating method. With traditional heating, heat is transferred to the reaction mixture through the vessel wall. This can cause localized overheating at the vessel walls, resulting in the formation of reaction side products and/or decomposition products, especially with prolonged heating. However, in MW heating, MW radiation passes through the walls of the (glass) reaction vessel and heats only the reactants and/or solvent, avoiding local overheating at the reaction walls. This can eliminate reaction side products and helps to explain the higher yields and purities often obtainable in MW-assisted syntheses in comparison to traditional methods, in often 1–10% of the time.7
MW reactors—graduating from the kitchen
The familiar domestic MW oven has seen the most use in synthesis so far due to its low cost and ready availability. However, the current trend is to the use of dedicated, commercial MW instruments, as these provide more homogeneous heating, reaction temperature control, built-in magnetic stirring, and significantly improved safety features.11 There are two types of commercial MW reactors—multimodal and monomodal systems. The multimodal system is most similar to the domestic MW oven; here MWs enter into the relatively large reaction chamber and are reflected by the reactor walls. The reflections of the waves generate a three-dimensional stationary pattern of standing waves in the cavity, called modes. The reaction vessel is commonly rotated in the reactor cavity so that it experiences a homogeneous field.In the other type of MW reactor, the monomodal system, the electric field is focused with a wave guide into a small reactor cavity where the reaction vessel sits. This cavity is sized such that only a single mode is present, which is believed to yield a more homogeneous distribution of energy within the cavity. Despite this purported benefit, multimodal reactors do have the advantage that numerous reactions can be performed simultaneously (i.e. in parallel) within the reaction chamber, while each reaction is typically performed sequentially in the smaller monomodal units.
Application of MW in solid-phase organic synthesis
Thermally demanding reactions, such as many Diels–Alder reactions, are often complete in hours in solution, but when performed on solid-phase can take multiple days, again due to the poorer reaction kinetics of heterogeneous reactions. If this solid-phase reaction is performed in the MW, however, the reaction rate often can be reduced from days to minutes! This level of acceleration has been observed by several research groups, and MW-assisted solid-phase reactions are being reported with increasing frequency. Just as solid-phase chemistry was first demonstrated with peptides, the first applications of MW irradiation to solid-phase reactions were peptide hydrolyses and couplings.12 Specifically, Yu et al. showed that polystyrene-bound peptides could be hydrolyzed in 7 min in a domestic MW, a process normally taking 24 h. Furthermore, traditional solid-phase peptide couplings were achieved in 4 min in 99–100% conversion with no detected racemization. Since these impressive reports 13 years ago, MW irradiation has been applied to a broad range of solid-phase reactions resulting in substantial rate acceleration, including Claisen and Knoevenagel condensations, nucleophilic substitutions, succinimide and hydantoin formation, and Suzuki couplings.7 As MW-assisted synthesis becomes more mainstream this list will increase, and numerous reactions that were deemed too sluggish to pursue on solid-support will likely be revisited.MW-assisted polymer-supported library synthesis
Given the success of several MW-assisted solid-phase reactions, the next critical step is to extend this enabling methodology to solid-phase combinatorial library synthesis. Indeed, over the past 7 years, there have been several parallel (but no split-pool) libraries prepared on polymeric supports where MW-assisted reactions have been employed as a key step.1 Although each library has been small (ranging from 5–96 members), these efforts have laid the foundation for the generation of larger libraries in the future. One notable example of these libraries, generated via a MW-assisted solid-phase multicomponent reaction (MCR), is shown in Fig. 2a.13![Examples of MW-assisted solid-phase reactions and combinatorial applications. a: Parallel library generated via Ugi-4CC reactions on amino-TentaGel resin.13b: Polystyrene-supported thionating reagent for the conversion of amides to thioamides.16c: Polystyrene-supported [1,3,2]oxazaphospholidene for the conversion of isothiocyanates to isocyanides.17d: Parallel library generated via Hantzsch-3CC reactions under solvent-free conditions (adsorbed onto NH4NO3–bentonite clay).18](/image/article/2003/OB/b301432k/b301432k-f2.gif) | ||
| Fig. 2 Examples of MW-assisted solid-phase reactions and combinatorial applications. a: Parallel library generated via Ugi-4CC reactions on amino-TentaGel resin.13b: Polystyrene-supported thionating reagent for the conversion of amides to thioamides.16c: Polystyrene-supported [1,3,2]oxazaphospholidene for the conversion of isothiocyanates to isocyanides.17d: Parallel library generated via Hantzsch-3CC reactions under solvent-free conditions (adsorbed onto NH4NO3–bentonite clay).18 | ||
MCRs, transformations where three or more reactants combine to give a single product, have received much attention due to their elegance, simplicity and overall efficiency in comparison to multi-step syntheses.14 Furthermore, MCRs can permit rapid entry into collections of diverse small molecules using sets of relatively simple and often commercially available building blocks. Thus, the application of MCRs in combinatorial library synthesis, such as Ugi, Bignelli, Hantzsch three- and four-component couplings (3 and 4CCs), has become progressively more popular, particularly in parallel approaches. Despite their elegance, however, many MCRs require prolonged heating for the reaction to proceed at an appreciable rate on solid-phase (e.g. the Ugi-4CC can take from 1 to 3 days to go to completion). To address this shortcoming, several research groups have investigated MW-assisted MCRs, both on polymer-bound reagents and impregnated inorganic solid supports (see below).
One example of a library synthesized via MW-assisted solid-phase MCRs is that of Hoel and Nielson, shown in Fig. 2a.13 The authors performed Ugi-4CC reactions in a monomodal MW reactor between polymer (TentaGel)-bound amines, and various aldehydes, carboxylic acids, and isocyanides to yield a “mini library” of 18 α-acylamino amides in just 5 min per compound! This represents an impressive three-orders-of-magnitude reduction in reaction time. While their yields were variable, the authors reported highly pure products (> 95%). Thus, the application of MW irradiation to solid-phase MCRs could permit these powerful transformations to achieve their predicted status as “workhorse” reactions for library synthesis.14 Also, as researchers look toward making larger solid-phase libraries in the future, MW-assisted reactions should soon find application in split-pool library synthesis.
Polymer-supported reagents for MW-assisted solution-phase synthesis
One recent twist on solid-phase organic synthesis is to attach covalently a reagent to the solid phase and then subject a solution of a chosen substrate to this supported reagent. The benefits of this “reverse” approach are numerous, including the ability to monitor the reaction as it proceeds using standard analytical techniques, the easy removal of toxic reagents and their by-products by simple filtration, and the ability to regenerate and re-use supported reagents. As with “traditional” solid-phase chemistry, however, reactions of solution-phase substrates with solid-supported reagents also proceed at a slow rate. Ley and co-workers have overcome this obstacle by employing MW irradiation in reactions mediated by their solid-supported reagents.15 Two elegant examples are shown in Fig. 2b and c, a polymer-supported thionating agent16 and a supported reagent for the conversion of isothiocyanates to isocyanides, respectively.17 Using a monomodal MW oven, both reactions gave highly pure products in excellent yields in a fraction of the time required with traditional heating (15–150 min vs. ∼30 h). To showcase the utility of this approach for library synthesis (Fig. 2c), the newly formed isocyanides, a valuable building block for several MCRs, were further processed in an Ugi-4CC to yield 18 novel bicyclic products.MW-assisted solvent-free library synthesis
All the MW-assisted reactions presented above have been conducted in polar organic solvents. With improper handling, the use of flammable organic solvents and reagents in MW reactors can be a significant safety hazard. To this end, numerous groups have explored solvent-free MW-assisted organic synthesis, where reagents are either adsorbed onto a polar, inorganic support (e.g. mineral oxides such as aluminas, silicas, clays and zeolites that readily absorb MW irradiation) or mixed and subjected to irradiation neat.7 Not only has this approach been heralded as safer, the reduced use of organic solvents also makes this a more environmentally responsible method, especially for large-scale reactions.Recently, this approach has been extended to parallel combinatorial library synthesis.1 One example is the solvent-free synthesis of a 96-member library of substituted pyridines via a one-step Hantzsch-3CC conducted in 96-well microtiter filter plates (Fig. 2d).18 Here, the β-keto ester and aldehyde reagents were impregnated onto a 5 : 1 bentonite clay–ammonium nitrate mixture (the ammonium nitrate serving as the source of ammonia). Irradiation for 5 min in a domestic MW oven, followed by washing of the product off of the support into a receiver “daughter” plate gave the 96 substituted pyridine products in > 70% purity overall. Notably, this library was also constructed and isolated from start to finish using robotics, showcasing how MW-assisted chemistry could be easily integrated into high-throughput, automated synthesis applications.
MW-assisted parallel library synthesis on planar supports
The application of MW irradiation to solid-phase chemistry has not been restricted to spherical polymer beads. Recently, large arrays of compounds have been synthesized on planar solid supports, including cellulose, polypropylene, and SiO2 TLC plates (Fig. 3). These techniques are noteworthy, as these planar supports not only allow for spatially addressable parallel library synthesis, but also permit immediate screening of reactions directly on the support after irradiation. | ||
| Fig. 3 MW-assisted library synthesis on planar solid supports. a: Solvent-free synthesis of N-substituted aryl piperazines on SiO2.19b: Schematic of thin layer chromatography (TLC) as a tool for parallel reaction screening in MW-assisted synthesis. c: MW-assisted parallel synthesis of 8000 1,3,5-triazine derivatives on planar cellulose support.20a | ||
In the case of SiO2 TLC plates, they can serve both as a support on which to perform synthesis and as a medium for simple chromatographic separation. Williams recently exploited this dual capability, synthesizing an array of N-substituted arylpiperazines by first spotting the neat reagents onto a glass-backed TLC plate and subjecting the plate to MW irradiation for 5 min in a domestic oven (Fig. 3a and b).19 After cooling, the reaction array was then eluted and visualized using UV light and chemical stains. No starting material was observed, and all the reactions cleanly gave one product. This approach was used to optimize systematically the reaction conditions for a range of substrates, after which syntheses were conducted on a larger scale by adsorbing the reagents onto bulk SiO2 gel. These larger scale reactions also gave good to excellent yields in very high purities (> 95%), in again just 5 min.
Researchers have also prepared planar library arrays in which the compounds are covalently attached to, as opposed to being adsorbed onto, the support. Scharn et al. have prepared arrays of 1,3,5-triazine derivatives on both cellulose and polypropylene membranes. In these studies, the compounds were chemically attached to the support via an acid cleavable linker, and the libraries were built up using the SPOT-synthesis technique (Fig. 3c).20 In one library array of peptide–1,3,5-triazine conjugates, the researchers found that a sluggish nucleophilic substitution reaction on monochlorotriazine intermediates, normally taking up to 4 days, could be dramatically accelerated under MW irradiation. Indeed, the reactions were complete after 6 min of irradiation in a domestic MW oven. Long reaction times are incompatible with the synthesis of compound macroarrays on planar surfaces due to fast evaporation of reactants. Therefore, the application of MW heating allowed the researchers to realize an 8000 member SPOT array, with reported conversions of 65–95%. Cellulose supports also facilitate many types of solid-phase binding assays and facile analysis of the library members by manually punching out the SPOT, cleavage, and elution. Thus, the combination of SPOT-synthesis with MW technology provides a highly versatile platform for library synthesis and compound screening applications.
Future outlook
MW-assisted reactions are certainly heating up solid-phase synthesis. While this is an abbreviated tour of the emerging field of MW-assisted combinatorial chemistry, I hope the selected examples have piqued the interest of the reader to further examine this rapidly evolving area of chemistry. As the field is in its infancy, its scope and limitations remain unknown. The data collected to date indicate, however, that MW-assisted reactions could have a significant future impact on combinatorial chemistry in several areas—these include: (1) split-pool library synthesis, (2) MCR based library synthesis, (3) automated synthesis, and (4) spatially addressable library synthesis on dual-purpose planar supports. I contend that MW-assisted organic reactions have the ability to transform the field of combinatorial chemistry on many levels—I look forward to the revolution.Notes and references
- For two recent reviews of microwave-assisted combinatorial chemistry, see:
(a) A. Lew, P. O. Krutzik, M. E. Hart and A. R. Chamberlin, J. Comb. Chem., 2002, 4, 95–105 CrossRef CAS
; (b) C. O. Kappe, Curr. Opin. Chem. Biol., 2002, 6, 314–320 CrossRef CAS
.
- R. B. Merrifield, J. Am. Chem. Soc., 1963, 85, 2149–2154 CrossRef CAS
.
- For reviews of combinatorial chemistry, see:
(a) R. E. Dolle, J. Comb. Chem., 2002, 4, 369–418 CrossRef CAS
; (b) F. Balkenhohl, C. Bussche-Hünnefeld, A. Lansky and C. Zechel, Angew. Chem., Int. Ed. Engl., 1996, 35, 2288–2337 CrossRef CAS
.
- Á. Furka, F. Sebestyén, M. Asgedom and G. Dibó, Int. J. Pept. Protein Res., 1991, 37, 487–493 CAS
.
- D. S. Tan and J. J. Burbaum, Curr. Opin. Drug Discovery Dev., 2000, 3, 439–453 Search PubMed
.
- T. Groth, M. Grøtli and M. Meldal, J. Comb. Chem., 2001, 3, 461–468 CrossRef CAS
.
- For a review of MW-assisted organic synthesis, see: P. Lidström, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225–9283 Search PubMed
.
- For a recent discussion of “non-thermal” MW effects, see: L. Perreux and A. Loupy, Tetrahedron, 2001, 57, 9199–9223 Search PubMed
.
- D. M. P. Mingos and D. R. Baghurst, Chem. Soc. Rev., 1991, 20, 1–47 RSC
.
- The second way MWs transfer energy, ionic conduction, results if there are free ions or ionic species present in the substance being heated. Here, ionic species also move with the oscillating electric field and again rapid heating is caused by the motion and resulting collisions of ions.
- Three main companies market MW reactors for organic synthesis, see: (a) Personal Chemistry AB, web address: www.personalchemistry.com. (b) Milestone Inc., web address: www.milestonesci.com. (c) CEM Corporation, web address: www.cemsynthesis.com.
- For the first reports of MW-assisted solid-phase reactions, see:
(a) H. M. Yu, S. T. Chen, S. H. Chiou and K. T. Wang, J. Chromatogr., 1988, 456, 357–362 CrossRef CAS
; (b) H. M. Yu, S. T. Chen and K. T. Wang, J. Org. Chem., 1992, 57, 4781–4784 CrossRef CAS
.
- A. M. L. Hoel and J. Nielson, Tetrahedron Lett., 1999, 40, 3941–3944 CrossRef CAS
.
- H. Bienaymé, C. Hulme, G. Oddon and P. Schmitt, Chem. Eur. J., 2000, 6, 3321–3329 CrossRef CAS
.
- For a recent perspective on new tools for organic synthesis, including supported reagents, see: S. V. Ley and I. R. Baxendale, Nat. Rev. Drug Discovery, 2002, 1, 573–586 Search PubMed
.
- S. V. Ley, A. G. Leach and R. I. Storer, J. Chem. Soc., Perkin Trans. 1, 2001, 358–361 RSC
.
- S. V. Ley and S. J. Taylor, Bioorg. Med. Chem. Lett., 2002, 12, 1813–1816 CrossRef CAS
.
- I. C. Cotterill, A. Y. Usyatinsky, J. M. Arnold, D. S. Clark, J. S. Dordick, P. C. Michels and Y. L. Khmelnitsky, Tetrahedron Lett., 1998, 39, 1117–1120 CrossRef
.
- L. Williams, Chem. Commun., 2000, 435–436 RSC
.
- D. Scharn, H. Wenschuh, U. Reineke, J. Schneider-Mergener and L. Germeroth, J. Comb. Chem., 2000, 2, 361–369 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2003 |
