Humidity-controlled mesostructuration in CTAB-templated silica thin film processing. The existence of a modulable steady state
Florence
Cagnol
a,
David
Grosso
a,
Galo J.de A. A.
Soler-Illia
a,
Eduardo L.
Crepaldi
a,
Florence
Babonneau
a,
Heinz
Amenitsch
b and
Clément
Sanchez
*a
aChimie de la Matière Condensée, UPMC-CNRS, 4 place Jussieu, 75252 Paris cedex 05, France. E-mail: clems@ccr.jussieu.fr
bInstitute of Biophysics and X-Ray Structure Research, Austrian Academy of Sciences, Steyrergasse 17/VI, 8010 Graz, Austria
First published on 25th November 2002
Abstract
A study is presented of the self-assembly process that takes place during CTAB-templated silica film formation through in situ SAXS, interferometry and water titration investigations during evaporation associated with dip coating under various conditions. This work shows that the quantity of water present in the film when the mesostructuration takes place depends on the relative humidity (RH) during deposition. Indeed, the system contains considerable quantities of water at high RH, while it loses water at low RH. The water content is demonstrated to be a critical parameter, as poorly ordered, 2D-hexagonal or 3D-cubic final structures are obtained, depending on the RH, in agreement with the general physico-chemical laws of CTAB mesophases. Furthermore, changing the RH or the solvent vapour pressure just after evaporation induces a film composition change and a potential mesostructure modification, evidencing a modulable steady state, during which the mesostructure can be modified by external influences. The present study pinpoints the role of processing conditions that are often considered secondary to chemical conditions. The conclusions of this study into the CTAB–TEOS system are also relevant to other surfactant-templated systems which undergo evaporation-controlled self-assembly.
Introduction
The preparation of surfactant-templated mesostructured silica thin films has received much attention because of their potential applications as sensors, optoelectronic devices, membranes, etc. They present a large surface area and a pore size distribution which is tailored by the surfactant size and structure through the template approach first introduced by Beck et al. for bulk homologous materials.1 Such materials are prepared by combining sol–gel chemistry with the texturation imposed by the physical chemistry of the surfactant and are deposited as thin films in a procedure called “evaporation-induced self-assembly”.2,3 This method has been applied to prepare silica and transition metal oxide films, using various inorganic precursors and types of surfactant.4–19 Nevertheless, because of the versatile properties of silica, most previous works focused on the cetyltrimethylammonium bromide–tetraethyl orthosilicate (CTAB–TEOS) system in acidic media, the former acting as the structure directing agent and the latter as the precursor. Various mesoporous structures have been obtained with this combination, including disordered (D), lamellar (L), 2D-hexagonal (P6m), 3D-hexagonal (P63/mmc) and cubic (Pm3n) structures.4–8,10–13 In these materials, the initial CTAB/TEOS ratio is often taken as the main critical parameter for tailoring the final mesostructure. Indeed, two-dimensional phases are generally observed for CTAB/TEOS > 0.15 (molar ratio), while three-dimensional phases are encountered for molar ratios below this value. In other words, the interfacial curvature associated with the packing parameter (g) values of the final hybrid mesostructure decreases with the VCTAB/(VCTAB + VSiO2) volume ratio, similarly to the effect of increasing CTAB concentration in pure solvents (dilution effect).20 As a general rule, if g < 1/3, the micelles are spherical, if 1/3 < g < 2/3, the micelles are cylindrical, and if g ≈ 1, lamellae are favoured (g = V/a0l, where V is the total volume of the surfactant chain, a0 is the effective head group area and l is the surfactant chain length).21The dynamic processes that occur during film deposition/evaporation were followed in real time by in situ small angle X-ray scattering (SAXS) and interferometry measurements, which show that the mesostructure is formed through a disorder-to-order transition, which may involve intermediate hybrid mesophases.22,23 CTAB–TEOS mesophase transitions have also been observed, but only for bulk materials prepared under basic and hydrothermal conditions.24,25 These results revealed that the self-assembly taking place under the latter conditions is highly co-operative between both the organic and inorganic phases through charge density matching variation, influencing the packing parameter, g. Indeed, the phase transitions are directly related to the chemical changes induced by the hydrolysis/condensation of TEOS in solution, which lasted a few hours in these studies. Such co-operation was also reported for thin film formation, where the condensation state of the silica moieties plays a critical role during self-assembly. The conventional sol–gel conditions that govern the hydrolysis/condensation rates (i.e. pH, concentration, sol ageing time, etc.) must, therefore, be precisely adjusted in order to fix the optimal extent of silica polymerisation before deposition.22 Consequently, the self-assembly taking place during dip coating is expected to occur through a similar co-operative mechanism, but the observed phase sequences are more likely due to the variation of the system composition induced by the evaporation. Indeed, silica thin films are usually prepared from alcoholic TEOS sols in which the condensation rate has been minimised by use of pH conditions that are close to the isoelectric point of silica (pH ≈ 2). Since thin film evaporation takes place in a few tens of seconds, one expects the evolution of the silica condensation to be negligible in such a short period of time and, therefore, the interaction between silica entities and CTAB to be influenced solely by the film composition. In addition, the film composition is constantly changing during deposition as a result of the volatile compound evaporation that takes place at the air–film interface. Therefore, the composition varies with time and locally in the film. Taking into account this distribution, it has been reported that the succession of all the structures matches with the general behaviour of surfactants in solution (i.e. decreasing micelle curvature through an increase in g with increasing surfactant concentration).20,23
Although both the CTAB/Si ratio and the degree of silica condensation are undoubtedly critical parameters that govern the final mesostructure, many other secondary, but correlated, parameters (e.g. composition of atmosphere, or dip coater size or operator, for instance) are never discussed. The present article focuses on the role of such often neglected processing parameters and, especially, on the influence of the environmental composition inside the dip-coating chamber. It will be demonstrated that they can also play a critical role and can make the mesostructure difficult to reproduce if not carefully controlled. To gain a better understanding of the effects of these parameters, the process of thin film deposition has to be described by dividing it into four main steps. (1) The preparation of the initial sol, for which the composition is fixed and which is subject to silica hydrolysis/condensation kinetics. (2) The evaporation of solvent and co-solvent during deposition. This is a very short step (a few tens of seconds), during which the film composition varies locally and with time. (3) The modulable steady state (MSS), which follows directly after the evaporation and corresponds to the point when the diffusion of volatile molecules equilibrates between the environment and film media. Here, the MSS depends mainly on the water and solvent vapour pressures. At this stage, further condensation of silica proceeds while the structure is established, but it can still be modified by external influences if the network is not too rigid. (4) The hybrid mesostructured xerogel film, which corresponds to the final state where the structure cannot be altered further as a result of extended condensation associated with a network that is too rigid.
In the present study, it will be shown that the third state (MSS) is very important because it is when the final structure is formed and when the network rigidifies. It is therefore crucial to understand what happens during this stage. As will be illustrated herein, films can be defined as hybrid liquid crystal-like systems for which the composition is fixed according to the external water and ethanol vapour pressures when the MSS is reached. A modification of the film composition is obtained if the RH, or the ethanol vapour pressure, are changed. If the silica matrix is still flexible enough, such compositional variation may induce a mesostructure modification. Here, we report that too long a period of time within an ethanol saturated atmosphere leads to the loss of mesostructuration, while the elimination of ethanol vapour combined with a precise adjustment of the RH allows a final mesostructure to be selected in the MSS, in agreement with the physico-chemical properties of CTAB in solution. More precisely, it will be demonstrated that at a high RH of 70%, the film reaches the MSS with a high quantity of water inside the film, which favours the formation of a discontinuous Pm3n cubic phase. On the other hand, a lower RH of 20% leads to lower quantity of water inside the film, which promotes the two-dimensional P6m mesostructure. An RH value that is too low may lead to poorly defined structures. The present work is highly important for the scientific community working with sol–gel-derived mesoporous thin films because an understanding of these effects and controlling such processing parameters will allow problems associated with the lack of reproducibility to be overcome. In addition, such humidity dependence can be extended to other systems, as has been observed for other surfactant–inorganic compositions undergoing the evaporation-induced self-assembly process.
Experimental
Initial sols and thin film preparation
The initial sols were prepared in two steps. First, a prehydrolysed TEOS sol was prepared by refluxing for 1 h TEOS, EtOH, HCl and H2O in the molar ratio 1∶3∶5 × 10−5∶1. CTAB was progressively added, together with EtOH, HCl and H2O, so that the final molar composition was 1 TEOS∶20 EtOH∶0.15 HCl∶5 H2O∶0.18 CTAB. The silica films were deposited from a aged sol aged for 3 days, for which condensation of the silica moieties had resulted in mainly Q2 and Q3 species, in 29Si NMR terms, as previously reported.22 The deposition was performed on 20 µm thick silicon wafers by dip coating at a constant withdrawal rate of 2.5 mm s−1 in a sealed cabinet, through which dry and/or water-saturated air was allowed to flow in a controlled proportion. The constant flow of this mixture was aimed at diluting and removing the ethanol vapour and adjusting the RH in the range 20 to 85%. In a typical experiment, air at controlled RH = 40% was allowed to flow within the dip coater for a few minutes and was stopped during deposition in order for the film evaporation to proceed without air convection. The flux was then resumed 60 s after deposition and maintained to the end of the experiment. For the three processes at RH = 20, 40 and 70%, the RH was fixed during the whole deposition, while for the RH = 40–85% process, the sequence was similar to that described for the RH = 40% process, except that the RH was increased up to 85% 10 min after deposition.Water titration
The content evolution of free water was estimated from parallel experiments in which 10 g of the initial sol was allowed to evaporate under a constant air flow of controlled RH (i.e. 20, 40 or 70%). The weight percentage of water was then measured by the Karl Fisher technique (Metrohm 701 KF Titrino), together with the global weight loss after different times of evaporation. The final water contents in these samples were assumed to be representative of those in the corresponding films in the MSS.Film characterisation
The self-assembly process during evaporation was studied by in situ time-resolved SAXS at the Austrian high flux SAXS beamline of the 2 GeV electron storage ring ELETTRA (Trieste, Italy). For such experiments, and contrary to the usual dip-coating process, the substrate remained fixed while the solution was lowered after immersion. This method ensured that the X-ray beam irradiated the same region of the film while the diffraction patterns were collected every 1 s on a 2D CCD detector. Simultaneously, the interference fringes, corresponding to the thickness variation induced by the progressive evaporation of the liquid phase, were recorded by interferometry. The last image of the interferometry investigation was used to assess the final homogeneity and optical quality of the film. Details of similar in situ SAXS/interferometry investigations have been described elsewhere.22The thickness of the as-prepared films, as measured by ellipsometry (SOPRA EF 4G), ranged from 350 to 400 nm.16 The film final structures were also confirmed by transmission electron microscopy (TEM; JEOL 100 CX II) after thermal treatment.
Results and discussion
As discussed in the introduction, in order to understand the self-assembly mechanism, better assessment of the evaporation and the modulable steady state is required. Fig. 1 shows the evolution of the film thickness deduced from the final thickness (measured by ellipsometry to be 400 nm for each process) and from the evolution of the interference fringes, a typical example of which is shown in the inset for the RH = 40% process. These results demonstrate that external water influences the evaporation process. The higher the applied humidity, the later the drying line (DL) appears (e.g. DL observed at 20, 25 and 65 s at RH = 20, 40 and 70%, respectively). Fig. 1 shows that the first stage of evaporation is similar for each process and is likely to be related to the preferential evaporation of alcohol.10,26 Indeed, the associated constant rate of evaporation in all explored cases, suggests that the departure of ethanol, being mainly dependent on its external relative vapour pressure, is not dramatically affected by the RH. A second regime of evaporation follows the ethanol departure at RH ≥ 40%. As shown in Fig. 2, where the evolution of the system water content is reported versus the progression of evaporation for each process, this second regime is associated with the diffusion of water inside and outside the film. At RH < 40%, water and ethanol simultaneously and constantly leave the film, while at RH ≥ 40%, water diffuses inside the film from the beginning of evaporation up to a maximum water content which is higher and located further along the evaporation line for higher RH. It is then followed by loss of water up to the MSS. This behaviour is qualitatively expected since, in the first regime, the film takes up water as a result of the constant ethanol evaporation and water diffusion from the environment to the film. When the film water content reaches the value at which it balances the relative vapour pressure of water (fixed by the RH), the trend is inverted and water starts to leave the film, leading to a decrease in the film water content. Therefore, the second regime is only observed for high RH conditions and is considered to be the water-rich period.26 The final water contents, measured by titration at the end of the evaporation experiments, are reported in Table 1. The system reaches a stable weight state with a higher water content for high RH than for low RH [e.g. H2O/Si (molar) = 7 at RH = 70% against 0.6 at RH = 20% and 4 at RH = 40%]. The final film water contents in the MSS are considered to be represented by these values, and consequently are higher for high RH than for low RH. | ||
| Fig. 1 Profile of the decrease in film thickness during evaporation at various relative humidities deduced from the final thickness, measured by ellipsometry, and from the fringes observed by interferommetry: RH = 20 (▲), 40 (●) and 70% (◆). The inset shows an example of typical interferometry fringes observed during evaporation at RH = 40%. The modulable steady state characterises the state were the film thickness does not decreases any more and where the mesostructure is established. | ||
 | ||
| Fig. 2 Film water content (H2O/Si molar ratio), deduced from Karl Fischer measurements, plotted versus the global film weight evolution during evaporation at RH = 20 (▲), 40 (●) and 70% (◆). The dashed line corresponds to the theoretical water content for which no water is either lost or gained by the system. | ||
| RH (%) | Final H2O/Si molar ratio | Intermediate phasesa | Final phase | Optical qualityb |
|---|---|---|---|---|
| a D, disordered; L, lamellar; 3DH, 3D-hexagonal (P63/mmc); C, cubic (Pm3n); 2DH, 2D-hexagonal (P6m). b T, transparent; NH, non-homogeneous. | ||||
| 20 | 0.6 | D, L | Undefined | T |
| 40 | 4 | D → 3DH → C → | 2DH | T |
| 70 | 7 | D → | C | NH |
| 40–85 | — | D → 3DH → C → 2DH → | C | T |
The second difference related to RH concerns the film organisation. Indeed, the final mesostructures, deduced from the 2D-SAXS patterns collected within the MSS, are reported in Fig. 3 and reveal that the RH = 40% process led to the 2D-hexagonal (P6m) phase characterised by the presence of the (10) in-plane diffraction. At RH = 20%, the structure appears to be composed of co-existing poorly resolved lamellar, cubic and 2D-hexagonal phases, as indicated by the three distinct in-plane diffractions and additional low intensity associated off-plane peaks (present in Fig. 3, but only observable through image analysis). At RH = 70%, a 3D-cubic phase was formed, as revealed by the (211) in-plane diffraction together with the relative off-plane peaks. Full indexations for each structure are reported in ref. 22. Since the three patterns are composed of diffraction dots and not rings, the domains are mono-oriented with the (211) direction of the cubic phase, the (10) direction of the hexagonal phase and the (001) direction of the lamellar phase classically oriented perpendicular to the film surface. The d-spacings deduced from these patterns are in the range 3.9 to 4.5 nm, and are in agreement with the characteristic lattice parameter values encountered in CTAB surfactant mesophases.20 Note that these d-spacings are not precisely reported here because the structures are constantly shrinking due to the progressive condensation of the silica framework. This mono-directional contraction has already been discussed in several works.13,21,27–29 Since three different hybrid structures were formed at three different RHs, it can be stated that the final water content is responsible for the formation of a specific mesostructure. This seems to be a general feature, since it has also been observed during self-assembly of mesostructured block copolymer-templated silica membranes.29
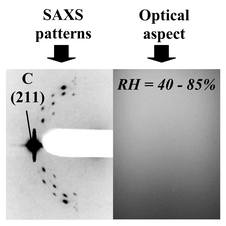
![2D-SAXS patterns, together with the corresponding interference images, of as-prepared films (in the modulable steady state) deposited at RH = 20, 40 and 70%. 2DH (P6m) and cubic (Pm3n) phases are obtained at RH = 40 and 70%, respectively. At RH = 20%, the pattern reveals the co-existence of poorly resolved lamellar, cubic and 2D-hexagonal phases. As a result of the epitaxial relation between the cubic and the 2D-hexagonal phase [related to the Pm3n
(211) and (12-1) peaks overlapping the P6m
(10) and (01) peaks, respectively],27 there is no evidence that only the cubic phase is represented at RH = 70%. The optical homogeneity at the macroscopic scale is given by the homogeneity of the interferometry image, since colour variation is due to any optical non-homogeneity.](/image/article/2003/JM/b209640b/b209640b-f3.gif) | ||
| Fig. 3 2D-SAXS patterns, together with the corresponding interference images, of as-prepared films (in the modulable steady state) deposited at RH = 20, 40 and 70%. 2DH (P6m) and cubic (Pm3n) phases are obtained at RH = 40 and 70%, respectively. At RH = 20%, the pattern reveals the co-existence of poorly resolved lamellar, cubic and 2D-hexagonal phases. As a result of the epitaxial relation between the cubic and the 2D-hexagonal phase [related to the Pm3n (211) and (12-1) peaks overlapping the P6m (10) and (01) peaks, respectively],27 there is no evidence that only the cubic phase is represented at RH = 70%. The optical homogeneity at the macroscopic scale is given by the homogeneity of the interferometry image, since colour variation is due to any optical non-homogeneity. | ||
The final interferometry images corresponding to the modulable steady state are also shown in Fig. 3. They illustrate that the higher humidity process, leading to the cubic structure, is accompanied by lower optical quality, characterised by the presence of defects and thickness non-homogeneity. This poor optical quality is not associated with the cubic structure. Indeed, it can be attributed to the longer second regime of evaporation reported in Fig. 1 that allows more time for the system to undergo secondary effects (i.e. phase separation, precipitation, edge effects, impurity adsorption, etc., which are usually encountered in thin film liquid deposition techniques). This is confirmed by the fact that optical transparency and the cubic structure were obtained by combining moderate and high humidity in the RH = 40–85% process. In this process, the evaporation takes place at RH = 40%. Thus, the second evaporation regime is short and leads to good thickness homogeneity. The humidity was rapidly increased up to of 85% 10 min after the deposition, which results in an increase in film water content (the extent of this increase could not be measured, but is confirmed by the lattice swelling described below). The corresponding final SAXS pattern and interferometry image in Fig. 4 suggest that cubic high structuration and optical quality are compatible and can be achieved using the latter combined process. More importantly, and as a first statement, this confirms that variation of the RH can still affect the mesostructure minutes after deposition, evidencing the modulable steady state, and that the cubic structure is favoured by the presence of water in the film.
 | ||
| Fig. 4 2D-SAXS pattern, together with the corresponding interference image, of an as-prepared film (in the MSS) deposited via the RH = 40–85% process. | ||
In order to get a better idea of what really drives the system to self-assemble into a specific supramolecular mesostructure, the structuration was followed in situ and in real time by SAXS. The different intermediate phases encountered during evaporation are reported in Table 1 and a typical phase sequence is shown in Fig. 5 for the RH = 40–85% process. As previously reported, the meso-organisation is formed via a disorder-to-order transition. Up until 15 s after deposition, no diffraction peak is observed. At 17 s, a poorly defined ring, characteristic of diffusion of isotropic micellar solutions [disordered (D)], appears. At 21 s, a typical 3D-hexagonal (3DH, P63/mmc) pattern was collected, suggesting that spherical micelles have rearranged into a three-dimensional compact structure, which is rapidly transformed into the intermediate cubic phase (C, Pm3n), itself turning into the 2D-hexagonal (2DH, P6m) final structure. Transition from one phase to the other lasts at least a few seconds, which means that phases can co-exist at the same time in the film as a result of local composition variation.23 The whole sequential transformation occurs over 100 s. In the first step (RH = 40%), the wet film dries homogeneously and ends up with excellent optical transparency. The corresponding structure in the MSS is 2D-hexagonal. When the RH is increased to 85% 10 min after deposition, water re-enters the system and the 2D-hexagonal → cubic Pm3n phase transition occurs via the epitaxial relation27 without perturbing the optical quality. Again, the corresponding lattice parameters are not mentioned here as they constantly varied following the swelling/contraction effect induced by the water diffusion. As a tendency, d(211) = 4.46 nm at 750 s, while d(10) = 3.84 nm at 100 s in Fig. 5. The phase sequence is D → 3DH → C → 2DH and follows what is expected considering the general behaviour of surfactants in solution (increasing surfactant concentration leads to less curved micelle interfaces and provokes the transition from spherical micelles to lamellae).20 The sequence observed for the RH = 20 and 70% processes showed only the disorder-to-order transition, without passing through the whole set of intermediate phases observed at RH = 40%, which could be related to the dynamics of evaporation. Indeed, evaporation which is too fast can prevent complete self-assembly and can thus quench the system within an intermediate state, as it has a shorter period of time to organise.13,28 Furthermore, this effect is enhanced by lower film water content, associated with low RH, which leads to high viscosity in the film, thus preventing facile molecular rearrangement.
 | ||
| Fig. 5 Time-resolved 2D-SAXS patterns, collected in situ during dip coating of films. Chronologically, air at RH = 40% is allowed to flow through the dip coater minutes before dip coating (DC, starting at t = 0 s). Evaporation then takes place in the absence of an air flow, in order to prevent any convection. The flow is then resumed 60 s after DC and maintained up to the end of the experiment for the constant RH = 40% process. In the RH = 40–85% process, the sequence is similar, except that the RH is increased to 85% 10 min after DC. DL stands for drying line. | ||
We have shown that the film water content is greatly influenced by the humidity (i.e. low humidity induces rapid departure of water from the film while higher humidity promotes diffusion of external water within the inorganic hydrophilic phase of the film). Here, the role of water is threefold. (i) The presence of water at the micelle–network interface promotes solvation/intercalation of H2O between the polar head groups, pushing them apart by steric hindrance, which results in an increase in the a0 dimension and, thus, a decrease in the g parameter. (ii) Increasing dilution through greater water content favours highly curved interfaces and low g parameters. (iii) Finally, this local dilution allows for the viscosity to be lowered, resulting in greater mobility of non-volatile species and facile surfactant supramolecular rearrangement. Consequently, a high final water content within the film in the MSS favours the formation of three-dimensional phases, such as the cubic Pm3n phase, associated with highly curved interfaces and low g parameters.20 According to the water titrations and under the sol–gel conditions employed here, the final molar ratios, r (r = H2O/CTAB), are 3, 22 and 40 for RH = 20, 40 and 70%, respectively. Assuming that the alcohol content is insignificant in the MSS, a poorly defined lamellar phase is obtained for r = 3, while a P6m phase is obtained for r = 22 and a cubic phase is obtained for r = 40. In Fig. 5, it can be seen that an intermediate cubic Pm3n structure is also formed between 20 and 30 s at RH = 40%. According to the water titration curve in Fig. 2, the water content is at a maximum before the MSS (e.g.rmax = 30, rMSS = 22). It is during this water-rich period that the intermediate cubic structure is formed. For pure H2O–CTAB compositions, Fontell et al. reported that the hexagonal P6m phase spans from 60 to 11 molecules of water per CTAB.20 From this, it can be seen that the g parameter decreases with r for both systems. On the other hand, the P6m phase is obtained in thin films for r values that are estimated to range from 10 to 30, which is less than what is required for pure CTAB–H2O systems. This is where the dilution effect has to be taken into account, since the film systems contain silica moieties with silanol groups that can act as extra water molecules. Unfortunately, no relation has been established so far between r, the quantity of silica moieties and the final mesostructure. In addition, no three-dimensional mesophase was reported in the ternary CTAB–EtOH–H2O diagram,20 suggesting that the hybrid cubic phase is formed through co-operative self-assembly.
The presence of water thus promotes the formation of discontinuous cubic structures, since they were observed as intermediate phases during the water-rich period, or as the final structure in the MSS. Fig. 6 summarises the various phases obtained and shows the corresponding TEM images. The transitions shown take place in the MSS and follow the theoretical variation of g discussed above. They concern the P6m and the Pm3n phases, between which an epitaxial relation exists, and has been reported for silica-templated systems.27 The theoretical energetic states of both phases are shown schematically in Fig. 7, where the two-dimentional P6m phase is less energetic for low quantities of water while the three-dimensional Pm3n phase is more stable at high water content. The energetic barrier that allows transformation of one phase into the other in the MSS requires an activation energy that depends on the viscosity of the system and, therefore, on the dilution and the extent of silica condensation. This is why a transition can no longer be initiated after a certain period of time in the MSS. Since the rate of condensation is higher for lower dilution, this MSS period is shorter for lower RH conditions.
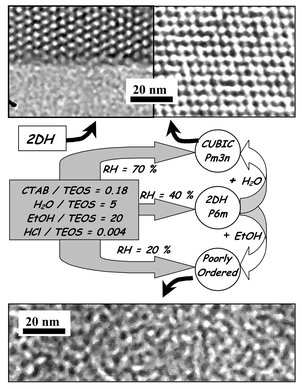 | ||
| Fig. 6 Scheme showing final film phases obtained for various RH, together with the corresponding TEM images. Phase transitions are provoked by water and ethanol vapour pressure variation when systems are in the MSS period. | ||
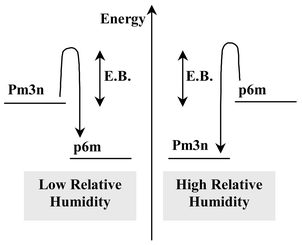 | ||
| Fig. 7 Theoretical energetic states of Pm3n and P6m structures stabilised in the MSS and for high and low humidity conditions. E.B. stands for the energetic barrier that corresponds to the activation energy necessary to allow phase transition (E.B. increases with the viscosity). | ||
Other types of water-dependent behaviour, relating phase transitions in similar MSS periods to external humidity, have also been observed for other systems. These are published elsewhere and concern non-ionic copolymer block templates combined with various inorganic matrices, such as SiO2,29 ZrO215 and TiO2.16,28 This suggests that the present study may be relevant for any templated hybrid materials prepared by evaporation techniques.
Conclusion
The present work clearly demonstrates the very important role of water from external humidity in the texturation of the hybrid mesostructure. Indeed, we have shown that conditions where the RH is too low prevent the formation of well-defined mesostructures, whereas high RH promotes the formation of a 3D-cubic structure through a high water content in the modulable steady state (MSS). In order to obtain both cubic structure and optical homogeneity and transparency, it is necessary to apply a moderate RH during the early stages of deposition and to allow the so-formed film to be aged in a more humid environment for a few minutes after deposition. This illustrates the second very important point concerning the possible modulation of the mesostructure during the MSS period by allowing volatile solvent or co-solvent to diffuse inside or outside the film. In the MSS, the mesostructure evolves with the chemical composition as in a ternary phase diagram, where the three components are the silicate intermediates, the water and the surfactant, assuming the viscosity is low enough to allow supramolecular rearrangement. Here, it is of utmost importance to emphasise the fact that the final mesostructure of a film is not only dependent on the sol composition and ageing time, but is also strongly influenced by the processing conditions. Evidently now, whatever the template–inorganic component combination, a structure may be different if there is no control on the environment. Furthermore, the present study can be extended to other systems composed of surfactant templates and inorganic precursors, and can be applied to other systems where the texturation is triggered by evaporation, such as oriented membranes29 or aerosol generation of particles.3,30,31Acknowledgements
Financial support from the French Ministry of Research, the CNRS, the CNPq (Brazil, grant no. 200636/00-0) and the Fundación Antorchas (Argentina) are gratefully acknowledged. The authors thank Anna Fischer for help with the in situ Karl Fischer measurements.References
- (a) J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS; (b) G. J. A. A. Soler Illia, C. Sanchez, B. Lebeau and J. Patariu, Chem. Rev., 2002, 102, 4093 CrossRef.
- C. J. Brinker, Y. Lu, A. Sellinger and H. Fan, Adv. Mater., 1999, 11, 579 CrossRef CAS.
- Y. Lu, H. Fan, A. Stump, T. L. Ward, T. Rieker and C. J. Brinker, Nature, 1999, 398, 223 CrossRef CAS.
- H. Yang, A. Kuperman, N. Coombs, S. Mamiche Afara and G. A. Ozin, Nature, 1996, 379, 703 CrossRef CAS.
- D. Y. Zhao, P. Yang, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Chem. Commun., 1998, 2499 RSC.
- D. Grosso, A. R. Balkenende, P. A. Albouy, M. Lavergne and F. Babonneau, J. Mater. Chem., 2000, 10, 2085 RSC.
- D. Grosso, A. R. Balkenende, P. A. Albouy, A. Ayral, H. Amenitsch and F. Babonneau, Chem. Mater., 2001, 13, 1848 CrossRef CAS.
- M. Klotz, P. A. Albouy, A. Ayral, C. Ménager, D. Grosso, A. Van der Lee, V. Cabuil, F. Babonneau and C. Guizard, Chem. Mater., 2000, 12, 1721 CrossRef CAS.
- D. Zhao, P. Yang, N. Melosh, J. Feng, B. F. Chmelka and G. D. Stucky, Adv. Mater., 1998, 16, 1380 CrossRef CAS.
- Y. Lu, R. Ganguli, C. A. Drewien, M. T. Anderson, C. J. Brinker, W. Gong, Y. Guo, H. Soyez, B. Dunn, M. H. Huang and J. I Zink, Nature, 1997, 389, 364 CrossRef CAS.
- S. Besson, T. Gacoin, C. Jacquiod, C. Ricolleau, D. Babonneau and J. P. Boilot, J. Mater. Chem., 2000, 10, 1331 RSC.
- M. Klotz, A. Ayral, C. Guizard and L. Cot, J. Mater. Chem., 2000, 10, 663 RSC.
- D. Kundu, H. S. Zhou and I. Honma, J. Mater. Sci. Lett., 1998, 17, 2089 CrossRef CAS.
- S. H. Tolbert, T. E. Schaffer, J. Feng, P. K. Hansma and G. D. Stucky, Chem. Mater., 1997, 9, 1962 CrossRef CAS.
- E. L. Crepaldi, G. J. A. A. Soler Illia, D. Grosso, P. A. Albouy and C. Sanchez, Chem. Commun., 2001, 1582 RSC.
- D. Grosso, G. J. A. A. Soler Illia, F. Babonneau and C. Sanchez, Adv. Mater., 2001, 13, 1085 CrossRef CAS.
- L. Pidol, D. Grosso, G. J. A. A. Soler Illia, E. Crepaldi, P. A. Albouy, H. Amenitsch, P. Euzen and C. Sanchez, J. Mater. Chem., 2002, 3, 557 RSC.
- M. Klotz, N. Idrissi-Kandri, A. Ayral and C. Guizard, Mater. Res. Soc. Symp. Proc., 2000, CC.7.4 Search PubMed.
- B. Lee, T. Yamashita, D. Lu, J. N. Kondo and K. Domen, Chem. Mater., 2002, 14, 867 CrossRef CAS.
- K. Fontell, A. Khan, B. Lindstrom, D. Maciejweska and S. P. Puang-Ngern, Colloid Polym. Sci., 1991, 269, 7 CrossRef.
- J. Israelachvili, Intermolecular and Surface Forces, Academic Press, New York, 2nd edn., 1992 Search PubMed.
- D. Grosso, F. Babonneau, P. A. Albouy, H. Amenitsch, A. R. Balkenende, A. Brunet-Bruneau and J. Rivory, Chem. Mater., 2002, 14, 931 CrossRef CAS.
- D. Grosso, F. Babonneau, G. J. A. A. Soler-Illia, P. A. Albouy and H. Amenitsch, Chem. Commun., 2002, 748 RSC.
- C. C Landry, S. H. Tolbert, K. W. Gallis, A. Monnier, G. D. Stucky, P. Norby and J. C. Hanson, Chem. Mater., 2001, 13, 1600 CrossRef CAS.
- S. H. Tolbert, C. C Landry, G. D. Stucky, B. F. Chmelka, P. Norby, J. C. Hanson and A. Monnier, Chem. Mater., 2001, 13, 2247 CrossRef CAS.
- M. H. Huang, B. S. Dunn and J. I. Zink, J. Am. Chem. Soc., 2000, 122, 3739 CrossRef CAS.
- S. Che, S. Kamiya, O. Terasaki and T. Tatsumi, J. Am. Chem. Soc., 2001, 123, 12089 CrossRef CAS.
- G. J. A. A. Soler-Illia, D. Grosso, E. L. Crepaldi, F. Cagnol and C. Sanchez, Mater. Res. Soc. Symp. Proc., 2002, 726, Q7–3 Search PubMed.
- G. J. A. A. Soler Illia, E. L. Crepaldi, D. Grosso, D. Durand and C. Sanchez, Chem. Commun., 2002, 2298 RSC.
- H. Fan, F. Van Swol, Y. Lu and C. J. Brinker, J. Non-Cryst. Solids, 2001, 285, 71 Search PubMed.
- D. Grosso, G. J. A. A. Soler-Illia, E. L. Crepaldi, B. Charleux and C. Sanchez, Adv. Funct. Mater., 2002, in press Search PubMed.
| This journal is © The Royal Society of Chemistry 2003 |
