Surfactant-assisted growth of uniform nanorods of crystalline tellurium
Zhaoping
Liu
a,
Zhaokang
Hu
a,
Qin
Xie
a,
Baojun
Yang
a,
Ji
Wu
a and
Yitai
Qian
*ab
aDepartment of Chemistry, University of Science and Technology of China, Hefei, Anhui, P. R. China 230026. E-mail: liuzp@mail.ustc.edu.cn; Fax: 86-551-3607402; Tel: 86-551-3603204
bStructure Research Laboratory and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, P. R. China 230026. E-mail: ytqian@ustc.edu.cn; Fax: 86-551-3607402; Tel: 86-551-3603204
First published on 14th November 2002
Abstract
Uniform tellurium single crystal nanorods with diameters of 14 nm and lengths of 300 nm were prepared through reduction of [TeS4]2− by SO32− with use of a suitable surfactant, sodium dodecyl benzenesulfonate (NaDDBS). Te nanorods with various diameters can be prepared by carefully adjusting the concentration of NaDDBS or the initial concentration of TeS42−. The nucleation and growth processes of Te nanorods were interpreted by solid–solution–solid transformation and surfactant-assisted growth mechanism.
Introduction
In the past decade, considerable attention has been drawn towards one-dimensional (1D) nanostructure materials, such as nanowires, nanorods and nanotubes, because of both their interesting physical properties and the wide range of their potential applications in nanodevices.1 Various approaches have been developed for the preparation of metal and semiconductor 1D nanostructure materials. Among them, template-directed synthesis,2 soft lithography,3 a laser ablation method,4 and the CVD (chemical vapour desorption) route,5 are considered as effective approaches. However, chemical approaches may provide a more promising technique to preparing 1D nanostructures than conventional methods in terms of cost and potential for large-scale production.6 Recently, a solution-phase method found favour for producing 1D nanostructure materials, such as Au nanorods,7 CdS nanorods,8 Ag nanowires,9 Se nanowires10 and Bi nanotubes.11Tellurium and related materials have attracted more and more attention.12 As a semiconductor, tellurium exhibits a unique combination of many interesting and useful properties,13 such as a relatively low band gap (∼0.33 eV),14 an effect of ultrafast electronic excitation on the A1 phonon frequency,15 and a high reactivity toward a wealth of chemicals that can be exploited to convert tellurium into other functional materials such as Bi2Te3,16 CdTe17 and HgCdTe.18 Presently, tellurium has modest usage in some non-ferrous alloys and was a secondary vulcanizing agent in the natural rubber industry. The availability of tellurium nanostructures with low dimensionalities should be able to bring in new types of applications or to enhance the performance of the currently existing device as a result of quantum-sized effects. Recently, ultrafine powders of acicular tellurium particles have been prepared by the γ-radiation method,19 and tellurium nanotubes with diameters over 60 nm have been synthesized by adding orthotelluric acid to pure ethylene glycol refluxed at 197 °C.20 Furthermore, other 1D nanostructures of tellurium with diameters over 50 nm have also been synthesized by a solution-phase, self-seeding method.21
In this paper, a simple precursor, ammonium sulftellurate ((NH4)2TeS4), was prepared initially. Then, a reduction reaction was employed in our synthesis of uniform Te single crystal nanorods through the reduction of (NH4)2TeS4 in an aqueous solution by sodium sulfite (Na2SO3), in the prescence of a suitable surfactant, sodium dodecyl benzenesulfonate (NaDDBS). The formation process of the nanorods was observed with TEM.
Experimental
Preparation of a simple precursor
(NH4)2TeS4 aqueous solution was prepared by the following procedure: analytically pure Te powder was added to an excess amount of aqueous ammonium polysulfide. The solution was heated to 80–90 °C while being stirred to dissolve the Te powder, and then a red solution containing (NH4)2TeS4 was obtained. The chemical reaction can be formulated as| Te + 3S22− → [TeS4]2− + 2S2− |
Preparation of Te nanorods
A 50 ml aqueous solution containing 20 mM (NH4)2TeS4 and 0.1 g NaDDBS was prepared in a conical flask, which was stirred with a magnetic stirrer and heated to 80 °C. Then, 30 ml of 0.1 M Na2SO3 solution was added dropwise into the mixture solution, under constant stirring. With the increase of the quantity of Na2SO3, the solution color changed from yellowish orange to pale yellow, and finally to black. After 10 min, the reaction solution was cooled to room temperature and then aged for 24 h. The black solution was stable in air and kept for more than one week without a precipitate. This mixture was centrifuged at 8000 rpm for 30 min to separate the Te nanorods. The solid portion was rinsed with distilled water and ethanol three times each.For comparsion, studies of various aging times (10 min, 30 min, 3 h and 12 h), a varying amount of NaDDBS (0.025 g and 0.4 g), or no NaDDBS, and differing initial concentrations of (NH4)2TeS4 (8, 35 and 50 mM) were also carried out.
Characterization
The samples were characterized by X-ray diffraction (XRD), employing a scanning rate of 0.05° s−1 in a 2θ range from 20° to 65°, using a Japan Rigaku D/max-γA X-ray diffractometer equipped with graphite monochromatized Cu Kα radiation (λ = 0.154178 nm). The morphology and particle sizes of the samples were determined by Hitachi 800 transmission electron microscopy (TEM) performed at 200 kV. The crystallinity of individual nanorods was analyzed with a JEOL 2010 high-resolution transmission electron microscope (HRTEM) running at 200 kV.Results and discussions
Uniform Te nanorods with diameters of 14 nm and lengths of 300 nm have been successfully synthesized using the present synthetic route. Fig. 1 shows the XRD pattern of the as-prepared sample obtained in a mixed aqueous solution containing 20 mM (NH4)2TeS4 and 0.1 g NaDDBS and being aged for 24 h at room temperature. All of the reflections of XRD pattern in Fig. 1 can be readily indexed with a pure hexagonal phase [space group: P3121(152)] of Te with lattice constants a = 0.4461 nm and c = 0.5880 nm, compatible with the literature values of a = 0.4458 nm and c = 0.5927 nm (JCPDS 36-1452). This XRD pattern indicates that pure Te products were obtained under current synthetic conditions. | ||
| Fig. 1 XRD pattern of the as-prepared sample obtained in a mixed aqueous solution containing 20 mM (NH4)2TeS4 and 0.1 g NaDDBS and being aged for 24 h at room temperature. | ||
Fig. 2 shows the TEM images and ED patterns of a respentative sample prepared under the same condictions as those presented in Fig. 1. TEM images of the samples taken from the resultant black solution show that nanorods are uniform in length (∼300 nm) and diameter (∼14 nm). Interestingly, it was found that these nanorods self-assembled in a manner resembling a two-dimensional nematic liquid crystal (Fig. 2A), and each nanorod was separated from the neighboring ones by about 1.5–2.5 nm (Fig. 2B), consistent with the presence of the surfactant molecules. The electron microdiffraction (ED) pattern (the inset of Fig. 2C) obtained from the individual nanorods confirmed that the Te nanorods are single crystalline. The diffraction spots could be indexed as the hexagonal phase of Te, with the longitudinal axis of each nanorod along the [001] direction. The HRTEM image shown in Figure 2C further supports our claim concerning the single-crystallinity of these Te nanorods. The fringe spacing (∼0.20 nm) observed in this image agrees well with the separation between the (003) lattice planes. The HRTEM image demonstrates that the nanorods have grown in a [001] direction.
 | ||
| Fig. 2 Representative TEM images (A and B) of the Te nanorods, and HRTEM image of an individual Te nanorods (C). Inset in (C) shows an ED pattern of individual Te nanorods. These Te nanorods were prepared under the same conditions as those presented in Fig. 1. | ||
We have systematically investigated the nucleation and growth process by collecting samples at different growth stages. Fig. 3 A–D show the TEM images of three sub-samples that were taken from the samples which had been aged at room temperature for different periods of time: (A) 10 min, (B) 30 min, (C) 3 h and (D) 12 h, respectively. After being aged for 10 min, a mixture of amorphous particles and rod-like particles can be observed (Fig. 3A). These nanorods have a diameter of ∼10 nm and a length of 50–100 nm, and their surface is very rough. It suggests that amorphous tellurium is formed at first. After 30 min, the length of these nanorods is over 100 nm and the crystal surface becomes smoother. However, nanorods having a diameter of ∼12 nm and a length of ∼300 nm appear at 3 h (Fig. 3C); they grow no more in length but a rough surface can still be observed. After being aged for 12 h, the diameters of these nanorods get to 14 nm and they have a smooth surface. Even with the aging time being prolonged (e.g. 24 h), the nanocrystals almost grow no more. From Fig. 3 A–D one can see that these nanorods grow along the longitudinal direction at a rate of ∼1.7 nm min−1, while the growth of their lateral dimensions is very slow. Our TEM studies suggested that solid-solution–solid transformation could be used to elucidate the growth mechanism of Te nanorods, which is similar to that of Se nanowires.22 In the first step, amorphous Te was formed in an aqueous solution through the reduction of Te(VI) at 80 °C. The chemical reaction we employed for synthesis of the Te nanorods can be formulated as
| [TeS4]2− + 3SO32−- → Te + S2− + 3S2O32− |
 | ||
| Fig. 3 Nucleation and growth of Te nanorods after being aged at room temperature for (A) 10 min, (B) 30 min, (C) 3 h and (D) 12 h, respectively. In this reaction system, 20 mM (NH4)2TeS4 and 0.1 g of NaDDBS were contained. | ||
Although there is a strong tendency toward 1D growth, we still believe that Te nanorod growth is surfactant-assisted. This is because NaDDBS is necessary in our experiment. In absence of NaDDBS, no nanorods formed (Fig. 4A). When the quantity of NaDDBS in the reaction system is increased to 0.025, 0.1 and 0.4 g, nanorods with a diameter of ∼30 nm, ∼14 nm and ∼7 nm, respectively, were obtained (see Fig. 4B, 2A, and 4C, respectively). However, the diameter of the rods would not decrease further even if 0.8 g of NaDDBS were added. These results indicate that only a small quantity of NaDDBS is needed for the growth of Te nanorods. It is well known that the presence of surfactant can influence the crystal growth. Controlling the shape of nanoparticles has been most successfully achieved using a surfactant template.23 It provides a constained environment during the nanoparticles growth and thus shapes are tuned according to the template. However, in the present reaction system, a rod-shaped micellar template should not be formed since only a small quantity of NaDDBS was added. With the assistance of NaDDBS, which could chemically absorb onto the surfaces of Te nanoparticles, the Te nanoparticles were able to grow into rod-shaped structures, as shown in Fig. 2. There are some similar examples showing that surfactant assists in controlling particle shape and size, such as PEG-assisted growth of Cu2O nanowires,24 PVP-assisted growth of Ag nanowires9 and NaAOT-assisted growth of Cu2S nanowires,25 but the mechanism is not completely understood. Here, we propose that the function of surfactant molecules should be to kinetically control the growth rate by interacting with these crystal faces of high energies through adsorption and desorption. Thus, at a higher concentration of NaDDBS in the reaction system, the lateral growth is restricted largely because the NaDDBS molecules may densely adsorb at the particle surface. A lower concentration of NaDDBS leads to a lower adsorbance of NaDDBS at the particle surface. As a result, the lateral growth is strengthened and the diameter of nanorods increases. Therefore, with the increasing concentration of NaDDBS, the diameter of the obtained nanorods decreases. On the other hand, the adsorption of NaDDBS at the Te nanorods surface appears to stabilize the rods. As a result, these nanorods self-assemble in a manner resembling a two-dimensional nematic liquid crystal.
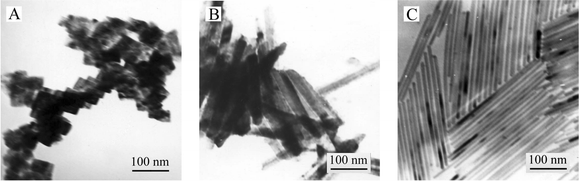 | ||
| Fig. 4 TEM images of Te products obtained by varying the quantity of NaDDBS: (A) in absence of NaDDBS, (B) 0.025 g NaDDBS and (C) 0.4 g NaDDBS. The initial concentration of (NH4)2TeS4 is 20 mM, and these samples were aged for 24 h at room temperature. | ||
Moreover, the initial concentration of (NH4)2TeS4 also has a significant effect on the diameter of the nanrods. Fig. 5 A–C show the TEM images of some 1D nanostructures of Te that were grown from other three solutions having different concentrations of (NH4)2TeS4. As the initial concentration of (NH4)2TeS4 was increased from 8 to 20, 35 and 50 mM, the diameters of the 1D nanostuctures of Te were changed from ∼30 to ∼14, ∼8 and ∼5 nm, respectively. On the other hand, when the initial concentration of (NH4)2TeS4 is over 35 mM, the aspect ratio of 1D nanostructures is higher than 50 and they can be seen as nanowires because of their curved shape. Thus, with increase of the initial concentration of (NH4)2TeS4, the diameter of the obtained 1D nanostructures decreases, and nanowires will be favorably formed under a higher initial concentration of (NH4)2TeS4.
 | ||
| Fig. 5 TEM images of Te products obtained at different initial concentrations of (NH4)2TeS4: (A) 8 mM, (B) 35 mM and (C) 50 mM. In each reaction system, 0.1 g NaDDBS was added. These samples were aged for 24 h at room temperature. | ||
Conclusions
In summary, crystalline Te nanorods have been synthesized by a reduction routine in the presence of the surfactant NaDDBS. Te nanorods with various diameters can be prepared by adjusting the concentration of NaDDBS or the initial concentration of (NH4)2TeS4. This reliable method can be controlled and is expected to be applicable to the preparation of 1D nanostuctures of other inorganic compounds. These uniform nanorods with high aspect ratio spontaneously form superstructures resembling nematic ordering. Thus, the semiconductor liquid crystal like structure may be used to fabricate electron and optical nanodevices. In addition, similar to Se nanowires,22 Te nanorods synthesized using this method can also be potentially converted into 1D nanostructures of other functional materials (such as CdTe, Bi2Te3 and Ag2Te) by reacting with appropriate elements or compounds.Acknowledgements
Financial support from the National Natural Science Fund of China and the 973 Project of China are appreciated.References
- (a) J. F. Wang, M. S. Gudiksen, X. F. Duan, Y. Cui and C. M. Lieber, Science (Washington, D.C.), 2001, 293, 1445 Search PubMed; (b) H. Kind, H. Q. Yan, B. Messer, M. Law and P. D. Yang, Adv. Mater., 2002, 14, 158 CrossRef CAS.
- (a) M. H. Huang, A. Choudrey and P. D. Yang, Chem. Commun., 2000, 12, 1063 RSC; (b) H. Q. Cao, Z. Xu, H. Sang, D. Sheng and C. Y. Tie, Adv. Mater., 2001, 13, 121 CrossRef.
- P. D. Yang, G. Wirnsberger, H. C. Huang, S. R. Cordero, M. D. McGehee, B. Scott, T. Deng, G. M. Whitesides, B. F. Chmelka, S. K. Buratto and G. D. Stucky, Science (Washington, D.C.), 2000, 287, 465 Search PubMed.
- (a) A. M. Morales and C. M. Lieber, Science (Washington, D.C.), 1998, 270, 1791 Search PubMed; (b) X. F. Duan and C. M. Lieber, Adv. Mater., 2000, 12, 298 CrossRef CAS.
- M. H. Huang, S. Mao, H. N. Feick, H. Q. Yan, Y. Y. Wu, H. Kind, R. Russo and P. D. Yang, Science (Washington, D.C.), 2001, 292, 1897 Search PubMed.
- Y. N. Xia, J. A. Rogers, K. Paul and G. M. Whitesides, Chem. Rev., 1999, 99, 1823 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Phys. Chem. B, 2001, 105, 4065 CrossRef CAS.
- L.-S. Li, J. Walda, L. Manna and A. P. Alivisatos, Nano Lett., 2002, 2, 557 CrossRef CAS.
- Y. G. Sun, B. Gates, B. Mayers and Y. N. Xia, Nano Lett., 2002, 2, 165 CrossRef CAS.
- B. Gates, Y. D. Yin and Y. N. Xia, J. Am. Chem. Soc., 2000, 122, 12582 CrossRef CAS.
- Y. D. Li, J. W. Wang, Z. X. Deng, Y. Y. Wu, X. M. Sun, D. P. Yu and P. D. Yang, J. Am. Chem. Soc., 2001, 123, 9904 CrossRef CAS.
- (a) Y. Park, J. Phys. Chem. Solids, 1999, 60, 1817 CrossRef CAS; (b) T. Yamaguchi, H. Ohtani and F. Yonezawa, J. Non-Cryst. Solids, 1999, 250-252, 437 Search PubMed; (c) B. C. Pan, Phys. Rev. B, 2002, 65, 085407 CrossRef; (d) S. Hunsche, K. Wienecke, T. Dekorsy and H. Kurz, Phys. Rev. Lett., 1995, 75, 1815 CrossRef CAS; (e) N. Takeuchi, Surf. Sci., 1999, 426, L433 CrossRef CAS; (f) Ph. Ott and J. R. Günter, Thin Solid Film, 2000, 366, 100 Search PubMed.
- L. Berg, V. Haase, I. Hinz, G. Kirschstein, H. J. Richter-Ditten and J. Wagner, Gmelin Handbook of Inorganic Chemistry, ed.G. Czack, D. Koschel and H. K. Kugler, Springer-Verlag, Berlin, 1983, vol. 8, pp. 1–223 Search PubMed.
- V. B. Anzin, M. I. Eremets, Yu. V. Kosichkin, A. I. Nadezhdinskii and A. M. Shirokov, Phys. Status Solidi A, 1977, 42, 385 CAS.
- P. Tangney and S. Fahy, Phys. Rev. B, 2002, 65, 054302 CrossRef.
- A. L. Prieto, M. S. Sander, M. S. Martin-Gonzalez, R. Gronsky, T. Sands and A. M. Stacy, J. Am. Chem. Soc., 2001, 123, 7160 CrossRef CAS.
- S. H. Song, J. Wang and M. Isshiki, J. Cryst. Growth, 2002, 236, 165 CrossRef CAS.
- K. J. Hong, J. W. Jeong, H. W. Baek, T. S. Jeong, C. J. Youn, J. D. Moon and J. S. Park, J. Cryst. Growth, 2002, 240, 135 CrossRef CAS.
- Y. J. Zhu, Y. T. Qian, H. Huang and M. W. Zhang, J. Mater. Sci. Lett., 1996, 15, 1700 CrossRef CAS.
- B. Mayers and Y. N. Xia, Adv. Mater., 2002, 14, 279 CrossRef CAS.
- B. Mayers and Y. N. Xia, J. Mater. Chem., 2002, 12, 1875 RSC.
- B. Gates, Y. Y. Wu, Y. D. Yin, P. D. Yang and Y. N. Xia, J. Am. Chem. Soc., 2001, 123, 11500 CrossRef CAS.
- (a) J. Lin, W. L. Zhou and C. J. O'Connor, Mater. Lett., 2001, 49, 282 CrossRef CAS; (b) N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389 CrossRef CAS; (c) L. M. Qi, J. M. Ma, H. M. Cheng and Z. G. Zhao, J. Phys. Chem. B, 2001, 105, 4065 CrossRef CAS.
- W. Z. Wang, G. H. Wang, X. S. Wang, Y. J. Zhan, Y. K. Liu and C. L. Zheng, Adv. Mater., 2002, 14, 67 CrossRef CAS.
- (a) S. H. Wang and S. H. Yang, Chem. Phys. Lett., 2000, 322, 567 CrossRef CAS; (b) S. H. Wang and S. H. Yang, Adv. Mater. Opt. Electron., 2000, 10, 39 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
