How hazardous are ionic liquids? Structure–activity relationships and biological testing as important elements for sustainability evaluation†
Bernd Jastorffa, Reinhold Störmanna, Johannes Rankea, Kerstin Mölterb, Frauke Stocka, Boris Oberheitmannc, Wolfgang Hoffmanncd, Jens Hoffmanne, Matthias Nüchtere, Bernd Ondruschkae and Juliane Filserb
aUFT Centre for Environmental Research and Environmental Technology, Department of Bioorganic Chemistry, Leobener Straße, 28359 Bremen, Germany. E-mail: jastorff@uni-bremen.de
bUFT Centre for Environmental Research and Environmental Technology, Department of General and Theoretical Ecology, Leobener Straße, 28359 Bremen, Germany
cUFT Centre for Environmental Research and Environmental Technology, Department of Epidemiology, Leobener Straße, 28359 Bremen, Germany
dErnst-Moritz-Arndt-Universität Greifswald, Institute for Community Medicine, Ellernholzstr. 1-2, 17487 Greifswald, Germany
eInstitute of Technical Chemistry and Environmental Chemistry, Friedrich-Schiller-University of Jena, Lessingstraße 12, 07743 Jena, Germany
First published on 10th March 2003
Abstract
For ionic liquids only few toxicological and/or ecotoxicological data are available until now. A strategy is presented which aims at an environmental risk assessment of chemicals, using a combination of structure–activity relationships (SAR), toxicological and ecotoxicological tests and modelling. The parts “test-kit-concept” and “multidimensional risk analysis” are described in detail by means of selected imidazolium ionic liquids. The iterative process of this strategy offers a tool for sustainable product design.
 Bernd Jastorff Bernd Jastorff | Prof. Dr. B. Jastorff was born in 1942. He studied chemistry at the University of Kiel starting in 1962 and obtained his PhD degree in 1970. Until 1973 he was research assistant at the Max-Planck-Institute for experimental medicine in Göttingen. Since 1973 he has been Professor at the University of Bremen, from 1996 to 2002 he served as Managing Director of the UFT Center of Environmental Research and Technology. His international collaborations led to guest professorships in The Netherlands, Poland and Romania, a Dr. h.c. from the University of Timisoara, Romania, and a Medal of Merit of the Medical University of Gdansk. He is head of the UFT Department of Bioorganic Chemistry, closely cooperating within the interdisciplinary UFT unit "Risk Research for Man and the Environment". |
| The development of ionic liquids as non-volatile solvents promises a group of solvents for synthesis whose dispersal into the environment should be more readily controlled and minimised. But how problematic are they if they do get into the environment? This article discusses the toxicity and ecotoxicity of ionic liquids using structure–activity relationships. Such tools can be used to aid the rational design of optimal solvents from a technical and environmental perspective. DJM |
Introduction
Ionic liquids are a fascinating group of new chemicals with the potential to improve development in organic chemistry and chemical technology,1,2 stimulating a lot of research fields. Over the past few decades the number of publications concerning ionic liquids has increased substantially.3 However, until now only few toxicological and/or ecotoxicological data are available for this group of chemicals. More information is needed to assess ionic liquids with regard to sustainability and to the principles of green chemistry. An adequate product design for this promising group of chemicals should consider not only technological needs but also toxicological and ecotoxicological risks. Fig. 1 depicts three ways to look at the structural elements of a substance. The “Technicophore” comprises structural elements which are mandatory for a technical application (e.g. function, synthesis, extraction). “Toxicophore” defines elements associated with risk for man and “Ecotoxicophore” correspondingly elements associated with risk for the environment.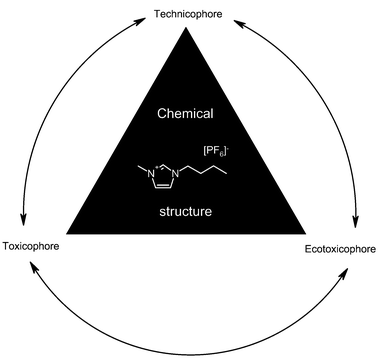 | ||
| Fig. 1 Aspects of structural elements of chemicals to be optimized within a process of sustainable product design. | ||
In an iterative process the technological needs have to be reconciled with a minimization of hazards for man and the environment. Here we report our strategy to identify the toxic and ecotoxicological potential of structural elements comprising ionic liquid structures and show the first results in the form of a preliminary comparative risk assessment of selected ionic liquids. Our strategy aims at an environmental risk assessment of chemicals, using a combination of structure–activity relationships (SAR), toxicological and ecotoxicological tests and modelling. Ionic liquids are a chemically diverse group of substances with a high potential for technical application which makes them an ideal model group for applying our strategy for sustainable design of chemicals.
In order to improve the data basis for the assessment of sustainability we formed a cooperation network including chemists, biologists, ecologists and epidemiologists. According to the “test-kit-concept” developed first for nucleotide analogues,4 specific compounds are synthesized in a first step. Furthermore physical and chemical properties of all these compounds are determined and toxicological as well as ecotoxicological data are generated utilizing test systems on different biological complexity levels (e.g. enzymes, cells, organisms, mesocosm studies).
1. A multidisciplinary working group has been established within the “Centre of Environmental Science and Environmental Technology (UFT)”. Due to cooperation with external partners the expertise was successfully extended (e.g. synthesis, physico-chemical and chemical properties, additional biological test systems).
2. To understand properties and activities of chemicals, we are using a rational approach of thinking in terms of structure–activity relationships (SAR).5
3. A theoretical prediction algorithm for presumed metabolites in biological systems is included in our approach.6
4. The “test battery ecotoxicity/toxicity” allows a systematic evaluation of (eco)toxicological properties of chemicals on biological systems of different complexity.
5. The findings from our own investigations are combined with information gained from literature in order to establish the process of multidimensional risk analysis.7,8
The interdisciplinary research unit “Risk Research for Man and the Environment” which is part of the “Centre of Environmental Research and Environmental Technology” (http://www.uft.uni-bremen.de) employs three infrastructural units:
1. SAR-QSAR
2. Analytics
3. Test battery ecotoxicity/toxicity
In the following, strategic concepts and first results obtained by these units are reported.
First the structural formula of a chemical has to be evaluated systematically to understand the structure–activity relationships. Applying an algorithm, the following properties (Table 1) can be obtained:
| 1 | Atom types present |
| 2 | Bond types present |
| 3 | Hybridisation of all atoms |
| 4 | Location of non-bonding electron pairs |
| 5 | Steric hindrance and conformational freedom |
| 6 | Possibility for geometric isomerism |
| 7 | Chirality |
| 8 | Ionic molecular interaction potential |
| 9 | Local dipole moments |
| 10 | Hydrophobic molecular interaction potential |
| 11 | Hydrogen bond donor potential |
| 12 | Hydrogen bond acceptor potential |
| 13 | π–π-bond interaction potential (charge transfer interaction potential) |
| 14 | Ring systems |
| 15 | Dissociating functional groups and estimated pKa-values |
| 16 | Functional groups which promote prototropic shift |
| 17 | All other functional groups and their potential reactions |
Evaluation of these properties opens the way to understand much of life in rational terms because it is expressed in the language of chemistry.9
Fig. 2 shows four important classes of cations for ionic liquids. It is obvious that the variability of the residues (R1, R2, R3, R4) in connection with the choice of different anions will result in a tremendous number of possible compounds. A theoretical analysis of these chemical structures already allows a differentiation (according to the ring structures, e.g. imidazolium-component versus pyridinium-component or different central atoms, e.g. phosphorus versus nitrogen).
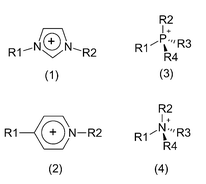 | ||
| Fig. 2 Four important classes of cations for ionic liquids: (1) imidazolium-, (2) pyridinium-, (3) phosphonium-, (4) ammonium-class. | ||
The first target class we concentrated on was imidazolium derivatives (Fig. 3) of ionic liquids which exemplify our way of analysis.
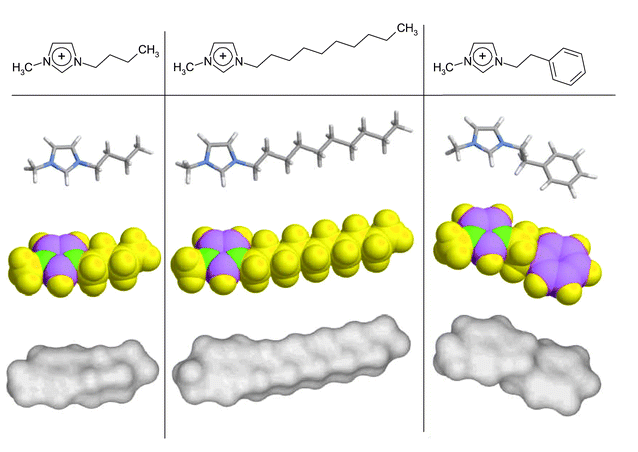 | ||
| Fig. 3 Selection of three imidazolium cations: from left to right: bmim, dmim, 2-phenylethyl-mim. The top row shows their structural formulas. In the next three rows features of their 3-dimensional structures (optimized with MOPAC 2000, PM3 method19) are shown: first row, stick models in CPK-coloration; second row, models in which the colours denote molecular interaction potential (yellow: hydrophobic interaction potential, green: positive charge, and violet: charge transfer potential5); third row, water-accessible surface of the cations.20 | ||
The central imidazolium ring is a delocalized aromatic system with high electron acceptor potential. Therefore, the nitrogen atoms are not able to form any hydrogen bonds. The system is very rigid and sterically inflexible. A methyl group in position R1 does not change this sterically stable state. In contrast, elongation of residue R2 (C4-chain, C10-chain) leads to a continuous increase of flexibility implying more conformational freedom. The R2-residue of 2-phenylethyl-3-methylimidazolium chloride ([2-phenylethyl-mim][Cl]) contains an additional aromatic system. The phenyl ring shows a high electron density including electron donor potentials. Lipophilic parts within this molecule are the alkyl group and the phenyl structural element but some lipophilicity also resides in the aromatic imidazolium ring.
All three selected compounds reveal the steric feature of a flat cation which results in flexibility and prevents direct and easy binding of polar compounds.
Within our systematic algorithm the prediction of possible metabolites has to be considered. According to this theoretical approach several points of action can be identified. If these ionic molecules actually reach the cytochrome P450 system located in the endoplasmatic reticulum of any cell, they can be oxidized in different positions of the alkyl side chains. The resulting metabolites can further be broken down metabolically to biocompatible fatty acids and imidazole (for part of this proposed metabolism for the 1-butyl-3-methylimidazolium ([bmim]) cation see Fig. 4)
![Theoretically predicted metabolism scheme for [bmim] cation (according to ref. 5).](/image/article/2003/GC/b211971d/b211971d-f4.gif) | ||
| Fig. 4 Theoretically predicted metabolism scheme for [bmim] cation (according to ref. 5). | ||
Of course it is possible that metabolites predicted theoretically may not be formed by nature. Nevertheless the prediction facilitates the analytical search for compounds while investigating the metabolic fate of ionic liquids in biological test systems. Such studies are under way within our cooperation network. Moreover, a computer supported expert system to predict metabolites of chemicals has been developed in collaboration with industry that might facilitate future work.10
Finally, two examples shall demonstrate the structure–activity relationship approach based on comparison of chemical structures. On the left side of Fig. 5, a pyridinium type of ionic liquid is presented in contrast to “Paraquat”. The herbicide “Paraquat” is considered to exhibit substantial toxicological and ecotoxicological risk (due to high acute toxicity and high persistence) and has therefore been banned.
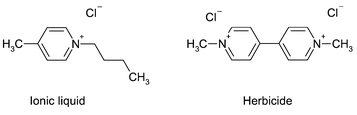 | ||
| Fig. 5 Comparison of 4-butyl-1-methylpyridinium chloride and the herbicide “Paraquat”. | ||
In the same way, 1-decyl-3-methylimidazolium chloride ([dmim][Cl]) and a patented plant growth regulator are depicted side by side in Fig. 6. Both examples indicate the structural similarity of some ionic liquids to already existing chemicals.
![Comparison of 1-decyl-3-methylimidazolium chloride ([dmim][Cl]) and a patented plant growth regulator.](/image/article/2003/GC/b211971d/b211971d-f6.gif) | ||
| Fig. 6 Comparison of 1-decyl-3-methylimidazolium chloride ([dmim][Cl]) and a patented plant growth regulator. | ||
Rationales for selection of test chemicals
Because of the high variability and indefinite number of possible ionic liquid species the choice of test compounds is difficult to perform. Therefore, we are using a so-called “test-kit-concept”.4 This concept has been used successfully over previous years to define nucleotide analogues as targets for systematic evaluation of enzymatic and cellular properties.11Starting from the chosen basic imidazolium structure, in general there are two possibilities to change residues. The first approach is to increase chain length in the R2-position keeping the R1-residue constant (methyl group). Secondly, we keep R2 constant but increase the length of R1 from methyl to ethyl. The third set of test compounds concentrated on imidazolium compounds with methyl groups in the R1-position and sidechains including a second aromatic ring (benzyl-, phenylethyl- and p-methylbenzyl moieties) in the R2-position. These considerations resulted in the test kits 1, 2 and 3 (Fig. 7). Last not least we systematically varied the anion leaving the positively charged moiety constant, leading to test-kit 4 (Fig. 7).
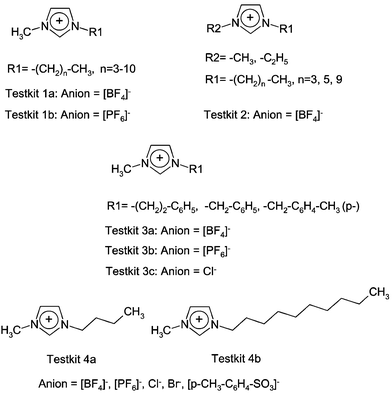 | ||
| Fig. 7 Compounds of the test kits 1, 2, 3 and 4. | ||
Rationales for selection of biological test systems
Due to the interdisciplinary approach described, a broad set of different methods to evaluate toxicological and/or ecotoxicological effects is available. Within the “Centre of Environmental Science and Environmental Technology (UFT)”, a test battery has been arranged that comprises genetic, cellular and sub-cellular toxicology as well as aquatic and terrestrial ecotoxicology. The latter two consist of various approaches, differing in organisms, endpoints and complexity.12 The test systems are selected according to regulatory requirements, questions under study and feasibility.In the first step chemicals are tested by rapid screening methods (e.g. cell viability assay, luminescence inhibition test with bacteria). Acute or sub-lethal screening methods and chronic tests representing different biological levels (Fig. 8) are available. Further tests are applied and designed on the basis of first results within a hierarchy of methods, starting with bacterial test systems and mammalian cell cultures up to complex mesocosms and human chromosomal aberration tests. All evaluated data will be compared to the effects predicted from SAR, including the metabolisation predictions.
 | ||
| Fig. 8 Levels of complexity for the evaluation of the biological activity of chemicals. | ||
First test battery results for different ionic liquid compounds of the chosen “test-kit” have already been generated. They will be explained in full detail in a different paper,13 but the main results are included in the first attempt of a risk analysis reported below, specifically in the section “Biological Activity”.
Multidimensional risk analysis
In the course of a case study about antifouling biocides, a multidimensional risk analysis based on five ecotoxicological indicators has been suggested.7 The indicators are Release (R), Spatiotemporal Range (S), Bioaccumulation (B), Biological activity (A) and Uncertainty (U). They have been defined and explained in detail.8The suggested multidimensional risk analysis is especially suitable for the comparison of chemical substances with a common scope of application. Since the imidazolium ionic liquids under investigation are being used as alternatives for conventional organic solvents, a first screening risk comparison of two selected substances with the common solvent acetone is presented here as a preliminary example. The chosen substances are 1-butyl-3-methyl-imidazolium tetrafluoroborate ([bmim][BF4]) and 1-decyl-3-methyl-imidazolium tetrafluoroborate ([dmim][BF4]), one for its abundance in organic synthesis literature, the other for its increased toxicity (see below). Each risk indicator is evaluated on a qualitative scale from 1 to 4, signifying a very low (1), rather low (2), rather high (3) and very high (4) risk. Additionally, the uncertainty of each evaluation is evaluated on a scale from very low (A), rather low (B), rather high (C) and very high (D). The scales are intended to be representative for solvents used in organic synthesis. The resulting five-dimensional risk profiles are depicted in Fig. 9.
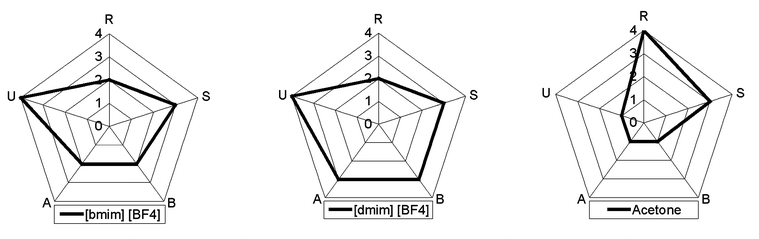 | ||
| Fig. 9 5-Dimensional risk comparison of two ionic liquids with acetone. High risk scores are located on the outside of the graph, low risk scores towards the centre. R = Release, S = Spatiotemporal Range, B = Bioaccumulation, A = Biological activity, U = Uncertainty. | ||
Release R
In order to provide a just risk comparison, only the application of acetone and imidazolium ionic liquids as solvents for organic synthesis is taken into account. Acetone in general is a substance with a very high production volume, greater than one million metric tons per year, but only a fraction is being used in organic synthesis. Because of its high volatility, its tendency to be released from technical systems is quite high. Contrarily, pure ionic liquids are practically non-volatile. Therefore, their release from technical systems via air can be assumed to be much smaller. Their limited solubility in water, however, results in a release via wastewater, potentially making biological treatment possible, while acetone in waste water will mainly be volatilized into air, the rest being mineralized. An additional pathway, not for the ionic liquids themselves but for their decomposition products, is evaporation of dealkylated imidazole derivatives. In summary, release of acetone is found to be high, with very low uncertainty (4A), while release of imidazolium ionic liquids [bmim][BF4] and [dmim][BF4] is expected to be rather low, with high uncertainty due to lack of information regarding decomposition reactions leading to volatilization and biodegradation in treatment facilities (2D).Spatiotemporal range S
The spatiotemporal range of the imidazolium ionic liquids is presumably governed by the interplay between water solubility, biotic and abiotic transformation and sorption to solid phases. No quantitative information about decomposition and biodegradation of any of the ionic liquids was accessible. Nevertheless it can be assumed that the [BF4] anions eventually undergo hydrolysis. Also, the imidazolium cations can be expected to biodegrade eventually, following pathways similar to the ones suggested above, but with unknown timeframe. Acetone will almost completely partition into air under environmental conditions. It is known to be decomposed by reaction with OH radicals and ozone in air with a half-life of ca. 10 to 22 days.14 Both properties in combination let its spatiotemporal range appear rather high, with rather low uncertainty (3B). The imidazolium cations also are likely to have a rather high spatiotemporal range, with a definitely very high uncertainty (3D).Bioaccumulation B
Acetone is highly volatile and readily biodegradable and therefore does not tend to bioaccumulate in the common sense. Therefore, the bioaccumulation of acetone from the environment is scored very low, with very low uncertainty (1A). The tendency of [bmim][BF4] and [dmim][BF4] to bioaccumulate is unknown. However, [dmim][BF4] can be presumed to have a tendency to be incorporated into membranes, because of its structural similarity to membrane lipids, comprising a charged head group with a nonpolar tail. Generally, the existing water solubility of the imidazolium tetrafluoroborates does not suggest high bioaccumulation. Bioaccumulation of [bmim][BF4] is scored rather low, with very high uncertainty (2D), and bioaccumulation of [dmim][BF4] is scored rather high, with equally high uncertainty (3D).Biological Activity A
A first study of biological activities of test kits 1, 2 and 4 has been conducted with three different test systems of the UFT test battery. Detailed results will be published elsewhere.13 A distinct influence of the length of the alkyl residues on toxicity was found. In all assays, ethyl imidazolium compounds were significantly more toxic than analogous methyl imidazolium compounds. Effect concentrations decreased by about 0.3 (IPC-81 leukemia cell lines) to about 0.6 (Vibrio fischeri luminescent bacteria) decadic log units for each additional carbon atom in the long n-alkyl chain. The biological activity of [dmim][BF4] is therefore estimated to be rather high, which is partially corroborated by some of the first results from terrestrial and aquatic plant tests; still with a rather high uncertainty, since the results from standard ecotoxicological tests are not available (3C). The biological activity of [bmim][BF4] is rather low, also with a rather high uncertainty (2C). In comparison, the biological activity of acetone is very low, in our own tests as well as in standard ecotoxicological tests, with very low uncertainty (1A).Uncertainty
From the above, the remaining uncertainty of the risk evaluation is clearly very low for acetone (1), but very high for both [bmim][BF4] and [dmim][BF4] (4). This shows that the risk comparison can only be very preliminary at this stage of knowledge. To compensate for this a clearly defined strategy to gather additional risk relevant information is inevitable and it would be suitable to apply such a strategy completely in advance, before ionic liquids become high production volume chemicals.Structure–activity relationships (SAR) and quantitative structure–activity relationships (QSAR) are powerful and rapid tools (Fig. 10) for a first theoretical assessment. Moreover, this approach allows us to define a clear chemical (synthesis and analysis), physical chemical (properties) and biological (effects on biological systems; see Fig. 8) experimental testing approach to check theoretical predictions and improve further predictions in an iterative way.
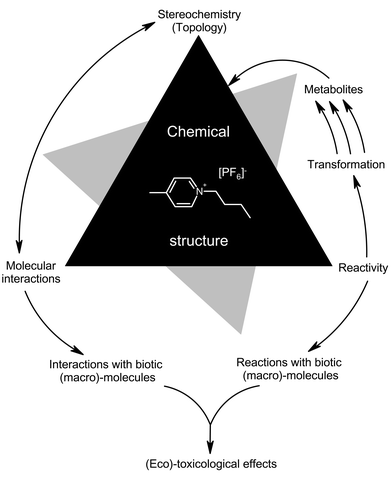 | ||
| Fig. 10 Structure–activity relationship (SAR) triangle.5 | ||
A strategy for sustainable product design
Sustainable development should bring about improvements in economical, ecological and social conditions for present and future generations. We agree with Diehlmann and Kreisel,15 stating that these considerations can take place on a local, regional or global level, which are all important.Looking at the sustainable development of ionic liquids, the integration of companies from the very beginning will open up the chance to consider technological needs while avoiding hazardous structures in the early steps to development of new industrial chemical products. Together, chemists, biologists and technologists have to find appropriate compounds based on the results of the SAR- and QSAR-algorithm. The structures of these compounds will be compared to each other and the properties will be assessed theoretically.4 In this step, all computer aided prediction systems can be integrated like toxicity (e.g. DEREK,16 TOPKAT17) and degradability prediction (e.g. EPIWIN18) or prediction of physico-chemical properties and others.19,20 This evaluation will lead to a selection of a first set of compounds to be synthesized. Afterwards the predicted properties have to be validated experimentally, i.e. technological requirements have to be met and toxicological and ecotoxicological testing have to be integrated as well. It is also necessary to consider economic issues (benefits and cost effectiveness) in order to yield a complete assessment integrating economic, social and ecological dimensions (Fig. 11) as demonstrated in the Agenda 21.21
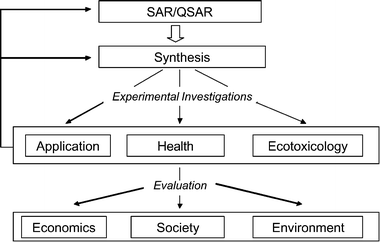 | ||
| Fig. 11 A conceptual framework for a sustainable design of chemicals, showing our current focus (adapted from8). | ||
Considering the whole life cycle of a chemical, waste treatment is another important aspect which has to be kept in mind. In close contact with the participating companies the field of application has to be evaluated, and differentiation between closed and open systems is necessary because the latter will implicate a broader release.
Conclusions
Using the iterative process described in this paper, the theoretical approach will help to reduce costs and to optimise the first and all following sets of compounds of interest. Although the procedure might appear very complicated and costly at first sight we propose to test its effectiveness. Our approach offers a promising tool to prevent undesirable effects of new technologies in advance of scaling up the production volume and may thus help to avoid remediation measures afterwards.22The costs of an iterative multi- and trans-disciplinary approach to sustainable product design can be reduced substantially by the use of SAR and QSAR and by a time- and work-sharing collaboration between industry and academic research. Using the example of designing ″green″ ionic liquids, we mainly aim at a continuous improvement of the strategy outlined here. For this purpose, our network is still open for further collaboration with other disciplines and people from academic research and from industry who are willing to add their expertise. We hope that ionic liquids in this way will become sustainable in the future.
Acknowledgements
Establishment and development of the “Test battery ecotoxicity/toxicity” is financially supported by the Senator for Women, Health, Youth, Social Affairs and Environmental Protection of the Freie Hansestadt Bremen.The discussion within the UFT directory board – Prof. Dr. Wolfgang Heyser, Prof. Dr. Norbert Räbiger, Prof. Dr. Dietmar Blohm, Prof. Dr. Armin Hildebrand, Prof. Dr. Rainer Frentzel-Beyme and Prof. Dr. Friedhelm Ventzke – contributed to the development of this concept.
References
- P. Wasserscheid, and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, Germany, 2002 Search PubMed.
- K. Seddon, Green Chem., 2002, 4, G35–G36 Search PubMed.
- R. D. Rogers and K. Seddon, Ionic Liquids: Industrial Applications for Green Chemistry, ACS Ser. 818, Oxford University Press, Oxford, UK, 2002 Search PubMed.
- B. Jastorff, E. G. Abbad, G. Petridis, W. Tegge, R. de Wit, C. Erneux, W. J. Stec and M. Morr, Nucleic Acids Res., Symp. Ser., 1981, 9, 219–223 Search PubMed.
- B. Jastorff, R. Störmann and U. Wölke, Struktur-Wirkungsdenken in der Chemie – eine Chance für mehr Nachhaltigkeit, Universitätsverlag Aschenbeck und Isensee, Oldenburg, Germany, 2003 Search PubMed.
- R. Störmann and B. Jastorff, Sci. Total Environ., Suppl., 1993, 1657–1667 Search PubMed.
- J. Ranke and B. Jastorff, Environ. Sci. Pollut. Res., 2000, 7, 105–114 Search PubMed.
- J. Ranke, Ecotoxicological Risk Profiles: Concept and Application to Antifouling Biocides, PhD Thesis, Berlin, 2002 Search PubMed.
- A. Komberg, Biochemistry,, 1987, 26, 6888–6891 CrossRef.
- J. Franzen, B. Jastorff, R. Störmann and A. Germanus, German Patent Application 10145904.1, 2002.
- F. Schwede, E. Maronde, H.-G. Genieser and B. Jastorff, Pharmacol. Ther., 2000, 87, 199–226 CrossRef CAS.
- T. Frische, Environ. Pollution., 2003, 121, 103–113 CrossRef CAS.
- J. Ranke, K. Mölter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffmann, B. Ondruschka, J. Filser and B. Jastorff, in preparation.
- GDCh-Advisory Committee on Existing Chemicals of Environmental Relevance (BUA), Acetone, S. Hirzel, Wissenschaftliche Verlagsgesellschaft, BUA reports 170, Stuttgart, Germany, 1997 Search PubMed.
- A. Diehlmann and G. Kreisel, Green Chem., 2002, 4, G15–G17 RSC.
- DEREK, v17.1, Lhasa Ltd., School of Chemistry, University of Leeds, Leeds, UK, 1998.
- TOPKAT, Health Designs Inc., Rochester, New York, USA, 1996.
- EPIWIN v3.10, Office of Pollution Prevention and Toxics, US EPA, 2000.
- J. J. P. Stewart, MOPAC 2000, Fujitsu Limited, Tokyo, 1999.
- MDL Chemscape Chime 2.6, MDL Information Systems, Inc., San Leandro, CA, USA, 1996–2001.
- See http://www.un.org/esa/sustdev/agenda21.htm.
- K. Hungerbühler, T. Mettier and J. Ranke, Chemische Produkte und Prozesse – Grundkonzepte zum umweltorientierten Design. Springer, Berlin, Germany, 1998 Search PubMed.
Footnote |
| † This work was presented at the Green Solvents for Catalysis Meeting held in Bruchsal, Germany, 13–16th October 2002. |
| This journal is © The Royal Society of Chemistry 2003 |
