Open Access Article
Open Access ArticleCarbazole-fused calixarene cavities: single and mixed AIEgen systems for NO detection†
Varun
Rawat‡
 a,
Abhishek
Baheti‡
a,
Om Shanker
Tiwari
a,
Abhishek
Baheti‡
a,
Om Shanker
Tiwari
 b and
Arkadi
Vigalok
b and
Arkadi
Vigalok
 *a
*a
aSchool of Chemistry, The Sackler Faculty of Exact Sciences, Tel Aviv University, Tel Aviv 69978, Israel. E-mail: avigal@tauex.tau.ac.il
bThe Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv 6997801, Israel
First published on 31st March 2023
Abstract
Novel oxygen-depleted calix[4]arenes containing fused carbazole moieties demonstrate AIEgen behavior in aqueous solutions. This phenomenon leads to highly sensitive detection of nitric-oxide guest molecules because it affects intra- and intermolecular energy transfer within aggregates.
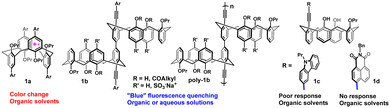
Nitric oxide (NO) is an important gaseous signaling molecule.1–3 Other diamagnetic members of this family can be conveniently detected by various fluorescent probes,4–7 but direct fluorescent detection of the radical NO represents a significant challenge due to its rapid oxidation to form higher nitrogen oxides. In fact, this oxidation is commonly used to indirectly measure the concentration of NO gas in biological systems.8 Direct detection by transition metal-based fluorescent NO probes has also been reported, but selectivity issues and compatibility with biological media remain.9–11 Rathore and colleagues, and later Rudkevich and co-workers, demonstrated that oxidized calix[4]arene (calixarene) scaffolds can trap the NO molecule within a hydrophobic cavity (1a, Fig. 1), accompanied by a color change,12–15 thus becoming supramolecular colorimetric NO probes. Such trapping is accompanied by charge transfer from NO to the oxidized host to give a stable diamagnetic donor–acceptor complex.15,16 We showed that conjugated 5,5′-bicalixarene hosts17 behave as fluorescent NO probes as they undergo blue fluorescence quenching by NO gas in organic solvents or in water, while insensitive to common gases (O2, CO, CO2) or ions (1b, Fig. 1).18,19 Incorporating these scaffolds into conjugated polymers led to a molecular wire-type signal amplification.19 Attachment of fluorophores at the termini of the conjugated fragment (Ar groups in 1b) gave access to new fluorescent scaffolds operating at longer wavelengths.20 However, using this approach to make water-soluble polymers emitting at longer wavelengths would add more steps to an already complex multistep synthesis. In addition, long wavelength-emitting poly(aryleneethynylenes) tended to exhibit diminished quantum yields in aqueous solutions.21
Thus, we decided to explore a different signal-amplification strategy for fluorescent supramolecular NO detection at longer wavelengths in aqueous media which was based on aggregation-induced emission (AIE).22 Recent studies have demonstrated the ability of guest intercalation to affect the luminescence properties of hosts exhibiting AIE.23–26 However, despite a great deal of attention in AIE-based sensing, such molecular probes utilizing a three-dimensional host–guest sensing mechanism are undeveloped in general,27 and for NO sensing, in particular.28,29 Here, we present the first examples of such supramolecular AIE-based probes for NO detection in aqueous solutions (Fig. 2).
Considering the weak emission response of small mono-calixarene compounds in organic solvents (1c,d, Fig. 1), we hypothesized that their aggregation in aqueous solutions could effectively increase the overall conjugation and, as a result, increase sensitivity. After the initial screening, we found that a carbazole-containing calixarene host 1c behaves as an AIE luminogen (AIEgen), showing strong AIE changes from blue emission in pure DMF solution to cyan (λem 450 nm) in a 60% water–DMF mixture. To our chagrin, the new aggregates did not show significant fluorescence changes after NO gas was passed through the solution for 5 min, implying weak interactions of the guest with a single aromatic ring of a calixarene cavity (ESI,† Fig. S52). To facilitate a stronger response, we chose to imbed an electron-rich polycyclic aromatic fragment into the calixarene cavity. It has been reported that polycyclic aromatic compounds interact with NO gas,30 but polycyclic conjugated calix[4]arenes are scarce and have not been employed in host–guest sensing.31–37
The synthetic routes toward compounds 2a and b incorporating a fused carbazole-calixarene unit are shown in Scheme 1a. Importantly, the calixarene intermediate 7 showed fluorescence in organic solvents, but an AIE effect in aqueous solutions was not observed until the phenolic oxygen was replaced by another fluorophore (compounds 2).
Unlike 1c, which showed a moderate fluorescence response upon bubbling of NO gas in a dichloroethane (DCE) solution, a solution of 2a demonstrated nearly complete quenching in the same solvent.38 More interestingly, when aggregated in an 80% water–DMF mixture, compound 2a exhibited strong fluorescence at longer wavelengths (λem = 495 nm). Contrary to 1c, upon the exposure to NO gas, aggregates of 2a demonstrated nearly complete fluorescence quenching (Fig. 3a). This observation provided evidence that the remote host–guest NO complexation had a pronounced effect on the fluorescent properties of the aggregates. In light of this encouraging fluorescence response, we decided to establish whether aggregates of 2a could be used in quantitative measurements of NO in aqueous solutions. To this end, we prepared stock solutions of a commercial NO source, diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (Et2N–N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O-Et2NH2+, DEA NONOate, 10), and studied the fluorescence response of aggregates of 2a to NO generated in situ. Gratifyingly, these aggregates showed very strong fluorescence quenching even at concentrations of 10 as low as 15 μM, 1.5 equiv. relative to 2a (Fig. 3b), which is within the concentration range found in human blood.39,40 A comparable sensitivity was observed previously for a conjugated polymer incorporating a water-soluble 5,5′-bicalixarene scaffold (poly-1b, Fig. 1).19 Thus, aggregation of 2a provided the fluorescence response to NO host–guest complexation on a par with the molecular wire-amplification mechanism, but at significantly longer wavelengths.
O)O-Et2NH2+, DEA NONOate, 10), and studied the fluorescence response of aggregates of 2a to NO generated in situ. Gratifyingly, these aggregates showed very strong fluorescence quenching even at concentrations of 10 as low as 15 μM, 1.5 equiv. relative to 2a (Fig. 3b), which is within the concentration range found in human blood.39,40 A comparable sensitivity was observed previously for a conjugated polymer incorporating a water-soluble 5,5′-bicalixarene scaffold (poly-1b, Fig. 1).19 Thus, aggregation of 2a provided the fluorescence response to NO host–guest complexation on a par with the molecular wire-amplification mechanism, but at significantly longer wavelengths.
A combination of an electron-rich cavity with an electron-poor conjugated fluorophore can lead to probes exhibiting intramolecular effects through transfer of bond energy.41,42 5,5′-bicalixarene scaffolds containing a combination of donor–acceptor fluorophores have demonstrated a ratiometric response to NO gas in organic solvents.20 Interestingly, while compounds 1d or 1b (Aryl = naphthalimide, Fig. 1)20 did not show fluorescence quenching upon passing NO through their DCE solutions, the fluorescence intensity of a carbazole-fused calixarene 2b containing an electron-poor naphthalimide fluorophore decreased by ∼37% from the original value under identical conditions.38 Similar to 2a, compound 2b gave aggregates in water–DMF mixtures (Fig. 3c). Upon exposure to NO, generated in situ from 1.5 equiv. of 10, these aggregates showed ∼33% of fluorescence quenching (Fig. 3d). This decrease in intensity was accompanied by a noticeable blue-shift of emission, indicating loss of donor–acceptor interaction between the electron-rich cavity and naphthalimide fluorophore. A carbazole or naphthalimide fluorescent moiety at the lower rim was essential to observe AIE in the carbazole-fused calixarenes 2a and b, so these flat fragments likely had a key role in aggregate formation.
However, the presence of a calixarene fragment was also crucial because an AIE effect was not observed for simple arylacetylene-substituted carbazole or naphthalimide compounds. Thus, we envisioned the formation of co-aggregates incorporating both, donor and acceptor, groups upon mixing of 2a and 2b in an 80% water-DMF. Such co-aggregates could exhibit strong donor–acceptor π-stacking and longer wavelength emission due to intermolecular energy transfer.43 In the presence of NO, host–guest interactions between the guest and the cavity of a donor (2a) would lead to attenuation of such intermolecular energy transfer and emission shift to shorter wavelengths. Indeed, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2a and 2b in 80% water–DMF demonstrated the properties expected from strongly interacting donor–acceptor aggregates. In particular, excitation in the carbazole absorption region (345 nm) led to a weak emission signal from the carbazole fluorophore, whereas the naphthalimide signal became stronger at a slightly longer wavelength (560 nm vs. 550 nm in pure 2b aggregates, Fig. 4a and 3c). For comparison, the same excitation of a 1
1 mixture of 2a and 2b in 80% water–DMF demonstrated the properties expected from strongly interacting donor–acceptor aggregates. In particular, excitation in the carbazole absorption region (345 nm) led to a weak emission signal from the carbazole fluorophore, whereas the naphthalimide signal became stronger at a slightly longer wavelength (560 nm vs. 550 nm in pure 2b aggregates, Fig. 4a and 3c). For comparison, the same excitation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2a and 2b in pure DMF solution showed the expected two separate signals for the carbazole fluorophore and naphthalimide fluorophore.38 These findings indicated intermolecular energy transfer between the donor (carbazole) and acceptor (naphthalimide) taking place upon aggregation. Importantly, the largest red-shift and highest fluorescence intensity was obtained when 2a and 2b were mixed in a 1
1 mixture of 2a and 2b in pure DMF solution showed the expected two separate signals for the carbazole fluorophore and naphthalimide fluorophore.38 These findings indicated intermolecular energy transfer between the donor (carbazole) and acceptor (naphthalimide) taking place upon aggregation. Importantly, the largest red-shift and highest fluorescence intensity was obtained when 2a and 2b were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. 4a) suggesting that ordered co-aggregate structures involving electron-donating and electron-accepting fluorophores were formed.43 When these co-aggregates were mixed with 10, substantial fluorescence quenching (70% with 7.5 equiv. of 10) and blue-shift of the remaining fluorescence was observed (Fig. 4b). The residual fluorescence intensity was similar to that obtained from the partly quenched fluorescence of 2b aggregates in an 80% water–DMF mixture (cf.Fig. 3d). These data indicated that NO complexation fully quenched the fluorescence of the electron-rich 2a, thereby making it incapable of transferring energy to 2b in a co-aggregate, and also partially quenched the fluorescence from the electron-poor 2b (vide supra). To obtain further information on the energy-transfer process in calixarene AIE co-aggregates, we prepared calixarenes 3a and 3b, isomeric to 2a and 2b, respectively (Scheme 1b). The carbazole-fused calixarene scaffold in compounds 3 contained the heterocyclic nitrogen atom in the para-position to the lower-rim fluorophore, allowing more efficient conjugation with this donor group than in compounds 2. Because of that, the emission of 3b was red-shifted noticeably compared with that of 2b in organic solvents and a water–DMF mixture (e.g., 585 nm for 3bvs. 550 nm for 2b in 80% water-DMF). Stronger donor–acceptor interactions in 3b also make it more responsive to the presence of low quantities of NO. For example, addition of 1.5 equiv. of 10 in an 80% water–DMF mixture led to ∼70% quenching of fluorescence of the aggregate (Fig. 5a), whereas only ∼33% was quenched in the aggregates of 2b (Fig. 3c). Similar to the isomeric 2, an 80% water–DMF solution containing a 1
1 ratio (Fig. 4a) suggesting that ordered co-aggregate structures involving electron-donating and electron-accepting fluorophores were formed.43 When these co-aggregates were mixed with 10, substantial fluorescence quenching (70% with 7.5 equiv. of 10) and blue-shift of the remaining fluorescence was observed (Fig. 4b). The residual fluorescence intensity was similar to that obtained from the partly quenched fluorescence of 2b aggregates in an 80% water–DMF mixture (cf.Fig. 3d). These data indicated that NO complexation fully quenched the fluorescence of the electron-rich 2a, thereby making it incapable of transferring energy to 2b in a co-aggregate, and also partially quenched the fluorescence from the electron-poor 2b (vide supra). To obtain further information on the energy-transfer process in calixarene AIE co-aggregates, we prepared calixarenes 3a and 3b, isomeric to 2a and 2b, respectively (Scheme 1b). The carbazole-fused calixarene scaffold in compounds 3 contained the heterocyclic nitrogen atom in the para-position to the lower-rim fluorophore, allowing more efficient conjugation with this donor group than in compounds 2. Because of that, the emission of 3b was red-shifted noticeably compared with that of 2b in organic solvents and a water–DMF mixture (e.g., 585 nm for 3bvs. 550 nm for 2b in 80% water-DMF). Stronger donor–acceptor interactions in 3b also make it more responsive to the presence of low quantities of NO. For example, addition of 1.5 equiv. of 10 in an 80% water–DMF mixture led to ∼70% quenching of fluorescence of the aggregate (Fig. 5a), whereas only ∼33% was quenched in the aggregates of 2b (Fig. 3c). Similar to the isomeric 2, an 80% water–DMF solution containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3a and 3b gave co-aggregates with the strongest emission and red-shift than solutions prepared with the other ratios, indicating ordered structures. This co-aggregate showed emission at 585 nm, ∼25 nm red-shifted compared with the 1
1 mixture of 3a and 3b gave co-aggregates with the strongest emission and red-shift than solutions prepared with the other ratios, indicating ordered structures. This co-aggregate showed emission at 585 nm, ∼25 nm red-shifted compared with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-aggregate of 2a and 2b. When the former was treated with a 15-μM solution of 10 (1.5 equiv.), nearly 60% fluorescence quenching was observed, with the remaining fluorescence signal undergoing a blue shift of 10 nm (Fig. 5b). In comparison, only 43% fluorescence quenching was observed for the co-aggregate of 2a and 2b under identical conditions. These results showed that, by tuning the donor–acceptor properties of the peripheral three-dimensional host, it was possible to further This tuning influenced the fluorescence properties of supramolecular aggregates. leading to more efficient utilization of intra- and intermolecular energy transfer. Incorporation of the NO guest within the cavity of 3a affected the intermolecular energy transfer between 3a and 3b in the co-aggregate, whereas additional NO complexation within the cavity of 3b further diminished the intramolecular energy transfer between the carbazole–calixarene cavity and naphthalimide fluorophore (Fig. 6a). Normalized emission spectra of the aggregates of 2a and b and 3a and b and their corresponding 1
1 co-aggregate of 2a and 2b. When the former was treated with a 15-μM solution of 10 (1.5 equiv.), nearly 60% fluorescence quenching was observed, with the remaining fluorescence signal undergoing a blue shift of 10 nm (Fig. 5b). In comparison, only 43% fluorescence quenching was observed for the co-aggregate of 2a and 2b under identical conditions. These results showed that, by tuning the donor–acceptor properties of the peripheral three-dimensional host, it was possible to further This tuning influenced the fluorescence properties of supramolecular aggregates. leading to more efficient utilization of intra- and intermolecular energy transfer. Incorporation of the NO guest within the cavity of 3a affected the intermolecular energy transfer between 3a and 3b in the co-aggregate, whereas additional NO complexation within the cavity of 3b further diminished the intramolecular energy transfer between the carbazole–calixarene cavity and naphthalimide fluorophore (Fig. 6a). Normalized emission spectra of the aggregates of 2a and b and 3a and b and their corresponding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures demonstrated higher sensitivity of compounds 3 to NO, especially at low concentrations (Fig. 6b).
1 mixtures demonstrated higher sensitivity of compounds 3 to NO, especially at low concentrations (Fig. 6b).
To further investigate the aggregation properties of carbazole-fused calixarenes, we undertook scanning electron microscopy (SEM). Accordingly, samples of 3b were dissolved in two solvents (80% H2O–DMF and in 100% DMF) at the same concentration (10 μM). The resulting solutions were drop-cast on a silicon wafer, and the aggregation properties of both samples were examined by SEM. Compound 3b exhibited highly aggregated particles in the sample drop-cast from 80% H2O–DMF (Fig. 7a), whereas negligible aggregation was observed in the SEM image of the sample obtained from pure DMF solvent (Fig. 7b).
Overall, we showed that the fluorescence of aqueous AIEgen aggregates could be affected by the host–guest complexation occurring at the peripheral conjugated cavity, as demonstrated by the detection of NO gas at micromolar concentrations. A combination of an electron-rich and electron-poor AIEgens led to ordered co-aggregates which allow shifting the analyte detection to longer wavelengths using intra- and intermolecular energy transfer.
This work was supported by the Israel Science Foundation grant (1328/20). We thank Professor Ehud Gazit for help with SEM measurements.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- H. Prast and A. Philippu, Prog. Neurobiol., 2001, 64, 51 CrossRef CAS PubMed.
- C. Bogdan, Nat. Immunol., 2001, 2, 907 CrossRef CAS PubMed.
- J. Garthwaite, Eur. J. Neurosci., 2008, 27, 2783 CrossRef PubMed.
- M. Yang, J. Fan, J. Du and X. Peng, Chem. Sci., 2020, 11, 5127 RSC.
- D. Andina, J.-C. Leroux and P. Luciani, Chem. – Eur. J., 2017, 23, 13549 CrossRef CAS PubMed.
- N. Kumar, V. Bhalla and M. Kumar, Coord. Chem. Rev., 2013, 257, 2335 CrossRef CAS.
- J. Krämer, R. Kang, L. M. Grimm, L. De Cola, P. Picchetti and F. Biedermann, Chem. Rev., 2022, 122, 3459 CrossRef PubMed.
- E. M. Hetrick and M. H. Schoenfisch, Annu. Rev. Anal. Chem., 2009, 2, 409 CrossRef CAS PubMed.
- M. H. Lim and S. J. Lippard, Acc. Chem. Res., 2007, 40, 41 CrossRef CAS PubMed.
- J. Alday, A. Mazzeo and S. Suarez, Inorg. Chim. Acta, 2020, 510, 119696 CrossRef CAS.
- For general issues with the fluorescent detection of NO in biological systems see: A. K. Vidanapathirana, P. J. Psaltis, C. A. Bursill, A. D. Abell and S. J. Nicholls, Med. Res. Rev., 2021, 41, 435 CrossRef CAS PubMed.
- R. Rathore, S. V. Lindeman, K. S. S. P. Rao, D. Sun and J. K. Kochi, Angew. Chem., Int. Ed., 2000, 39, 2123 CrossRef CAS PubMed.
- R. Rathore, S. H. Abdelwahed and I. A. Guzei, J. Am. Chem. Soc., 2004, 126, 13582 CrossRef CAS PubMed.
- G. V. Zyryanov, Y. Kang, S. P. Stampp and D. M. Rudkevich, Chem. Commun., 2002, 2792 RSC.
- E. Wanigasekara, C. Gaeta, P. Neri and D. M. Rudkevich, Org. Lett., 2008, 10, 1263 CrossRef CAS PubMed.
- The resulting adduct is better viewed as a charge transfer complex between the nitrosonium cation (NO+) and an electron-rich aromatic ring: R. Rathore, S. V. Lindeman and J. K. Kochi, J. Am. Chem. Soc., 1997, 119, 9393 CrossRef CAS.
- For the original synthesis of 5,5′-Bicalixarenes see: P. Neri, A. Bottino, F. Cunsolo, M. Piattelli and E. Gavuzzo, Angew. Chem., Int. Ed., 1998, 37, 166 CrossRef CAS.
- A. Molad, I. Goldberg and A. Vigalok, J. Am. Chem. Soc., 2012, 134, 7290 CrossRef CAS PubMed.
- B. B. Ahuja and A. Vigalok, Angew. Chem., Int. Ed., 2019, 58, 2774 CrossRef CAS PubMed.
- A. Baheti, R. Dobrovetsky and A. Vigalok, Org. Lett., 2020, 22, 9706 CrossRef CAS PubMed.
- X. Zhao, M. R. Pinto, L. M. Hardison, J. Mwaura, J. Müller, H. Jiang, D. Witker, V. D. Kleiman, J. R. Reynolds and K. S. Schanze, Macromolecules, 2006, 39, 6355 CrossRef CAS.
- Z. Zhao, H. Zhang, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9888 CrossRef CAS PubMed.
- L. Xu, Z. Wang, R. Wang, L. Wang, X. He, H. Jiang, H. Tang, D. Cao and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9908 CrossRef CAS PubMed.
- P. Wei, Z. Li, J.-X. Zhang, Z. Zhao, H. Xing, Y. Tu, J. Gong, T. S. Cheung, S. Hu, H. H.-Y. Sung, I. D. Williams, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Mater., 2019, 31, 1092 CrossRef CAS.
- Y.-Y. Chen, X.-M. Jiang, G.-F. Gong, H. Yao, Y.-M. Zhang, T.-B. Wei and Q. Lin, Chem. Commun., 2021, 57, 284 RSC.
- D. Dai, J. Yang and Y.-W. Yang, Chem. – Eur. J., 2022, 28 Search PubMed.
- M. Gao and B. Z. Tang, ACS Sens., 2017, 2, 1382 CrossRef CAS PubMed.
- K.-W. Lee, H. Chen, Y. Wan, Z. Zhang, Z. Huang, S. Li and C.-S. Lee, Biomaterials, 2022, 289, 121753 CrossRef CAS PubMed.
- J. Qi, L. Feng, X. Zhang, H. Zhang, L. Huang, Y. Zhou, Z. Zhao, X. Duan, F. Xu, R. T. K. Kwok, J. W. Y. Lam, D. Ding, X. Xue and B. Z. Tang, Nat. Commun., 2021, 12, 960 CrossRef CAS PubMed.
- M. N. Möller and A. Denicola, Biol. Med., 2018, 128, 137 Search PubMed.
- P. Lhoták, Org. Biomol. Chem., 2022, 20, 7377 RSC.
- M. Tlusty, H. Dvorakova, J. Cejka, M. Kohout and P. Lhotak, New J. Chem., 2020, 44, 6490 RSC.
- W. Hüggenberg, A. Seper, I. M. Oppel and G. Dyker, Eur. J. Org. Chem., 2010, 6786 CrossRef.
- R. Miao, Q.-Y. Zheng, C.-F. Chen and Z.-T. Huang, J. Org. Chem., 2005, 70, 7662 CrossRef CAS PubMed.
- O. G. Barton, B. Neumann, H.-G. Stammler and J. Mattay, Org. Biomol. Chem., 2008, 6, 104 RSC.
- F. Elaieb, D. Sémeril, D. Matt, M. Pfeffer, P.-A. Bouit, M. Hissler, C. Gourlaouen and J. Harrowfield, Dalton Trans., 2017, 46, 9833 RSC.
- S. Chowdhury and P. E. Georghiou, J. Org. Chem., 2002, 67, 6808 CrossRef CAS PubMed.
- See ESI† for the details.
- Y. Yoon, J. Song, S. H. Hong and J. Q. Kim, Clin. Chem., 2000, 46, 1626 CrossRef CAS.
- E. L. Kanabrocki, M. George, R. C. Hermida, H. L. Messmore, M. D. Ryan, D. E. Ayala, D. A. Hoppensteadt, J. Fareed, F. W. Bremner, J. L. H. C. Third, P. Shiraz and B. A. Nemchausky, Clin. Appl. Thromb./Hemostasis, 2001, 7, 339 CrossRef CAS PubMed.
- C.-W. Wan, A. Burghart, J. Chen, F. Bergström, L. B.-Å. Johansson, M. F. Wolford, T. G. Kim, M. R. Topp, R. M. Hochstrasser and K. Burgess, Chem. – Eur. J., 2003, 9, 4430 CrossRef CAS PubMed.
- D. Cao, L. Zhu, Z. Liu and W. Lin, J. Photochem. Photobiol., C, 2020, 44, 100371 CrossRef CAS.
- Naphthalimide and carbazole co-aggregation has very recently been reported in the design of photoredox catalysts: H. Lin, J. Wang, J. Zhao, Y. Zhuang, B. Liu, Y. Zhu, H. Jia, K. Wu, J. Shen, X. Fu and X. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202117645 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc01181j |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2023 |