A passive sampling method to determine ammonia in ambient air
Lotti
Thöni
*a,
Eva
Seitler
a,
Andreas
Blatter
b and
Albrecht
Neftel
b
aFUB–Research Group for Environmental Monitoring, C.P. 1645, CH-8640 Rapperswil, Switzerland. E-mail: fub@active.ch; Fax: +41 55 211 05 56; Tel: +41 55 211 05 55
bFAL–Swiss Federal Research Station for Agroecology and Agriculture, CH-8046 Zürich-Reckenholz, Switzerland
First published on 2nd January 2003
Abstract
Ambient ammonia concentrations, mainly originating from agricultural activities, have increased in the last few decades in Europe. As a consequence, critical loads on oligotrophic ecosystems such as forests and mires are greatly exceeded. Monitoring of ambient ammonia concentrations is necessary in order to investigate source–receptor relationships. Measuring ambient ammonia concentrations continuously with high time resolution is very expensive and cost-efficient systems are required. Where time resolution is of minor importance, several cost-effective systems, mainly dry denuder and passive samplers, can be applied. In this paper the Zürcher passive sampler, a diffusive sampling system, is presented. It is a Palmes type sampler with an acidic solution as absorbent and is easy to handle. It was tested at 46 sites in Switzerland over one year. The average concentration in ambient air was 2.5 µg m−3 ± 0.4 µg m−3. The average of the blank values were 0.21 µg m−3. The detection limit (double the standard deviation of the blank values) was 0.36 µg m−3. Three passive samplers were exposed at each site and each period. The mean standard deviation of these triplicate measurements was 9.5%. Compared with a discontinuous tubular denuder system and a continuous annular denuder system, the deviation was less than 10%. The Zürcher passive sampler is a useful and cost-efficient tool to determine long-term average ammonia concentrations (one- to four-week periods) in ambient air for mean concentrations above 1 µg m−3.
1. Introduction
In many natural ecosystems in Europe such as forests and bogs the critical loads of nitrogen are exceeded. Ammonia is recognised as one of the major sources of nitrogen deposition. To continuously monitor ambient ammonia concentrations, sophisticated measurement systems like AMOR1 are necessary. They are very expensive and labour intensive and can, therefore, only be used in a small number of sites in Europe. For many purposes weekly or monthly integrated measurements are sufficient. For measurements over a long period of time and in a larger area, passive diffusive samplers are well suited. They are easy to handle and cost-efficient. Dry denuder2,3 systems combined with an active sampling system are more labour intensive and logistically more demanding, e.g. they need electric current.A new generation of passive samplers of the Palmes type4 was originally designed by Fritz Zürcher at EAWAG (Swiss Federal Institute for Environmental Science and Technology) and then developed 12 years ago at the former Swiss Federal Research Station for Agricultural Chemistry and Environmental Hygiene (FAC, now incorporated into the Federal Research Station for Agroecology and Agriculture, FAL–Zürich-Reckenholz), for emission measurements.5 FUB improved the system so that low ambient air concentrations could be measured as well. Most of the Palmes samplers rely on an irreversible uptake of ammonia on a coated surface. The Zürcher passive sampler described in this paper uses another approach. Ammonia diffuses through a porous membrane into an acidic absorption solution. The main benefits are: first, it excludes as much interference as possible due to the absorption of ammonium-containing aerosols, and second, it avoids the extraction step of the coated surfaces in the samplers.
The Zürcher passive samplers were exposed for one year from autumn 1999 till autumn 2000 on 46 sites in Switzerland, including at city stations, in agglomerations, in regions with intensive and extensive agriculture, in forests, and in mountainous areas without human activities. Three samplers per box were exposed at one place, and they were changed usually every two weeks. Thus it was possible to apply the Zürcher passive samplers on a wide range of emission situations and throughout all seasons and compare the passive samplers with more sophisticated systems.
2. Materials and method
Fig. 1(a) shows a diagram of the Zürcher passive sampler. It is a polypropylene cylinder with a cover and a Teflon gasket on the upper side and an opening at the bottom. For transport and storage, the opening is covered. The Teflon membrane (0.2 µm) is placed 7 mm above the edge of the tube. It separates the ambient air from the absorption liquid, which consists of a strongly diluted HCl solution (0.0016% v/v) and 1,2-ethanediol (20% v/v) as an anti-freeze. 2.5 to 3.5 ml of solution are put in a passive sampler. The solution can be directly measured e.g. with ion chromatography.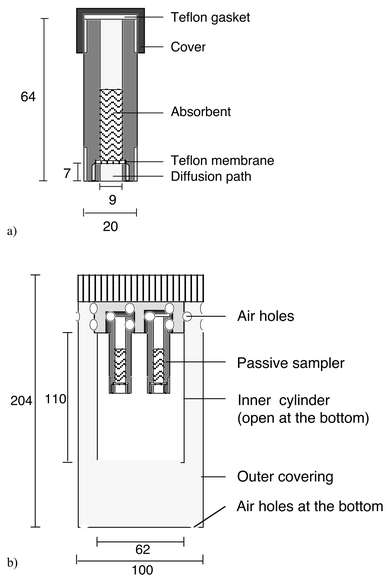 | ||
| Fig. 1 (a) The Zürcher passive sampler and (b) the shelter box for exposuring the samplers. Unit of measurement: mm. | ||
A special shelter box was constructed to eliminate air turbulence at the inlet of the passive sampler (Fig. 1(b)). This shelter is needed as the diffusion length is only 7 mm and as turbulence can shorten the diffusion length. The box consists of an inner cylinder, open at the bottom, surrounded by a closed box, with 21 air holes (diameter 9 mm) at the top of the side wall and seven at the bottom (six along the board and one in the centre). In the first experiments by Blatter et al.,5 the shelter box was open at the bottom covered with a membrane to prevent turbulent air. For conditions with relative humidity close or equal to 100%, a liquid film on the membrane develops that may absorb NH3. Not all of the ammonia reaches the absorption solution, especially in colder seasons, and the concentration in the air is usually underestimated. For exposure the boxes are attached to a pole or a similar device, normally 1.5 to 3 m above ground.
3. Calculation of the air concentration of ammonia
| J | NH3-flux in the diffusion path | µg m−2 s−1 |
| M | Mass intake | µg |
| ρ air | Mass concentration of NH3 in ambient air | µg m−3 |
| ρ s | Mass concentration of NH3 on the surface of the absorption solution | µg m−3 |
| ρ abs | Mass concentration of NH4+ in the absorption solution | µg NH3 m−3 |
| ρ bl | Mass concentration of NH4+ in blank | µg NH3 m−3 |
| T | Exposure time | s |
| V abs | Volume of absorption solution after exposure | m3 |
| A | Cross-section of diffusion opening | m2 |
| l D | Diffusion length | M |
| R | Total resistance | s m−1 |
| R D | Diffusion resistance | s m−1 |
| R M | Membrane resistance | s m−1 |
| D | Diffusion constant of NH3 in air (at 293 K, 1013 hPa) | m2 s−1 |
| T mean | Mean temperature over time exposed | K |
| T o | Standard temperature (293 K) | K |
| p o | Standard atmospheric pressure (1013 hPa) | hPa |
| p mean | Mean atmospheric pressure over time exposed | hPa |
 | (1) |
In this application ρair is of interest, eqn. (2). The absorption solution has to be chosen so that ρs ≈ 0:
 | (2) |
The diffusion resistance RD and membrane resistance RM are added to the total resistance R, eqn. (3):
 | (3) |
The diffusion resistance can be calculated with Fick's first law, eqn. (4):
 | (4) |
R M was determined experimentally.5 A calibration with various diffusion lengths gave a constant factor for the membrane resistance RM. RD and RM were calculated with linear regression, eqn. (5):
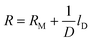 | (5) |
The axis intercept is RM, the reciprocal amount of D is the slope factor.
R was determined as 420 ± 10 s m−1. The percentage of the membrane resistance RM was 30% of the total resistance R (for lD = 7 mm). The experimental result for D was 23.6 mm2 s−1 (standard deviation ≤ ±10%). In the literature, the experimentally determined diffusion constants of NH3 in air (at 293 K, 1013 hPa) are 22.7 mm2 s−1,6 24.4 mm2 s−12,7 and 23.1 mm2 s−1.8 Fuller et al.9 calculated a diffusion constant of 23.6 mm2 s−1 using comprehensive data. The temperature dependence of the diffusion constant was experimentally determined as T1.42. This value was used to adjust the NH3 uptake to the mean temperature during the exposure period.
ρ air was corrected for mean temperature and pressure during the exposure period according to eqn. (6):
 | (6) |
4. Blank values, detection limit, precision, capacity
The following quality control results are based on samples from 46 sites taken during one year mostly at two-week intervals.Blank values: the measurement of ammonia is sensitive to contamination. Therefore handling in the laboratory is carried out in an environment with poor ammonia concentrations. This is achieved by placing a napkin saturated with citric acid in front of the mouth and nose while working and placing sheets of blotting paper and paper tissues also saturated with citric acid in the bags with the passive samplers during storage and transportation. Nevertheless, blank values were measured. The 90 blank results averaged 17.1 µg l−1 and ranged between 0.0 to 96.4 µg l−1 and had a standard deviation of 13.5 µg l−1. The blank values showed a normal distribution. 17.1 µg l−1 corresponded with 0.21 µg m−3 ammonia in the air (14 days exposition).
Detection limit: calculated from the blank values (twice standard deviation) 0.36 µg m−3 was for the two-week measurements.
Precision: to prevent loss of results, three samplers per box were exposed at each site and changed every two weeks. Of the total 2928 passive samplers, 66 (2.3%) lost liquid or had other field problems. 45 individual values (1.8%) were declared to be stray values, as they were twice as high or higher than the other two values in the same box and period. It was assumed that these stray values were due to unusual contamination during handling in the laboratory or in the field. From the 976 measurements with the three passive samplers each, with a total average value of 2.48 µg m−3, a standard deviation of 0.23 µg m−3 or 9.5% was computed.
Capacity: in winter 2002 three shelter boxes, each containing three Zürcher passive samplers, were exposed in the motorway tunnel “Gubrist” in Switzerland13 for eight days. In the first box samplers were changed four times (i.e. every two days), in the second twice (i.e. every four days) and in the third only once (Table 1). At least ten to hundred times higher concentrations than in open areas in Switzerland were measured there with the Zürcher passive sampler. The capacity limit was not reached within 8 days with a mean concentration of more than 200 µg m−3. In an earlier study (not published) a limit of 500 µg m−3 NH3 concentration over a one-week period was found.
| 19.2.2002 | 23.2.2002 | 4 days | 214 | |
| 23.3.2002 | 27.2.2002 | 4 days | 218 | 216 |
| 19.2.2002 | 27.2.2002 | 8 days | 212 | 212 |
5. Comparison with independent ammonia measuring systems: AMOR and denuder
For one year, beginning in August 1999, a comparison between the Zürcher passive samplers and a tubular denuder system2,3 was carried out in a garden area of Wallisellen, Switzerland, a town in the agglomeration of the city of Zurich (Fig. 2). The annual average of the passive sampler measurements was 2.18 µg m−3 and of the denuder 2.33 µg m−3. They corresponded well, taking into account the degree of precision of the two systems. | ||
| Fig. 2 Comparison of two independent ammonia measurement systems in Wallisellen, Switzerland. The denuder is an active sampling system. | ||
The Zürcher passive samplers were also compared with an on-line wet annular denuder system (AMOR1) and again with a batch denuder system2,3 (changed weekly) and with several other passive sampler systems.10 The Zürcher passive samplers were changed at 1 week, 2 week, and 4 week intervals. The passive samplers and the denuder were analysed in the same laboratory, but the detection of ammonia with AMOR was completely independent. The comparison took place in Aidling/Riegsee in Bavaria, Germany. Aidling is a village in a typical prealpine agricultural region. Fig. 3 shows that the agreement was good. Mean values over the whole measuring period were: AMOR: 3.83 µg m−3, denuder 3.62 µg m−3, passive sampler 1 week 3.69 µg m−3, 2 week 3.74 µg m−3 and 4 week 3.89 µg m−3.
 | ||
| Fig. 3 Comparison of three ammonia measurement systems in Aidling10 from June till December 1997. AMOR is a continuous measurement system, and the denuder an active sampling system. | ||
6. Examples of measurements
In Fig. 4 the development of ammonia concentrations in ambient air is shown for four sites with different emission patterns. Further results of the measurement campaign are described in Thöni et al.11 The values measured in winter, far away from ammonia sources, were near the detection limit (0.36 µg m−3), but in the warmer season the concentrations were also very low and the annual mean was 0.5 µg m−3. In the extensive agricultural area we also found very low values in the cold season, but higher values up to 4 µg m−3 during the times when cattle were put out to pasture and manure was brought out. The annual mean was 1.7 µg m−3. In a region with intensive agricultural activities we found high concentrations, on average 6.2 µg m−3, with large differences during the test year. The high concentrations found in the centre of a city directly beside a busy street had a mean value of 7.5 µg m−3. The high levels are due to motor traffic emissions,12 (the catalyst causes emission of ammonia) and perhaps emissions from human sewage and dogs' urine. | ||
| Fig. 4 Ammonia concentrations over one year at four stations in Switzerland with different emission patterns. | ||
7. Discussion and conclusions
The Zürcher passive sampler system is very useful for determining ammonia in ambient air with high levels of accuracy for mean concentrations above 1 µg m−3 for two week periods. When estimating lower concentrations, its measurements are not as accurate. The passive sampler system shows the seasonal fluctuations in the ammonia concentrations as well as the mean values. It is a low-cost method and the shelter box with the samplers can be mounted easily on a pole or a similar device. Various other passive samplers systems from other institutes have been tested in recent years, e.g. badge-type and Palmes-type samplers with coated surfaces, most of them with good results.10,14 Thijesse et al. for example tested Palmes tubes in the Netherlands with diffusion paths of 71 mm and 36.5 mm14 (Zürcher passive sampler 7 mm). They also fitted the reference measurements well, but only for higher concentrations than those measured in Switzerland. The main benefits of the Zürcher passive sampler is, that it avoids the extraction step of the coated surfaces in the samplers and it allows measurements of both low and high concentrations in air.Thus the Zürcher passive sampler can be recommended for measuring one-, two-, and four-week ammonia concentrations of 1 to 250 µg m−3 (two week means) in air but is less suited for concentrations below 1 µg m−3 (two week means).
References
- G. P. Wyers, R. P. Otjes and J. Slanina, Atmos. Environ., Part A, 1993, 27(13), 2085.
- M. Ferm, Atmos. Environ., 1979, 13, 1385–1393 CrossRef CAS.
- P. Alean and J. Hertz, in Proceedings of the Convention on Long-range Transboundary Air Pollution, EMEP-workshop of Measurements of Nitrogen-containing Compounds, Les Diablerets, Switzerland, 1992 Search PubMed.
- E. D. Palmes, A. F. Gunnison, J. DiMatti and C. Tomczyk, Am. Ind. Hyg. Assoc. J., 1976, 37, 570–577 CAS.
- A. Blatter, M. Fahrni and A. Neftel, CEC Air Pollut. Res. Rep., 1992, 41, 171–176 Search PubMed.
- S. P. S. Andrew, Chem. Eng. Sci., 1955, 4, 269–272 CrossRef CAS.
- B. A. Ivakin and P. E. Suetin, Sov. Phys.–Tech. Phys. (Engl. Transl.), 1964, 8, 748–751 Search PubMed.
- R. S. Brama, T. J. Shelly and W. A. McClenny, Anal. Chem., 1982, 54, 358–364.
- E. N. Fuller, P. D. Schettler and J. Calvin Giddings, Ind. Eng. Chem., 1966, 58, 19–27 Search PubMed.
- M. Kirchner, S. Braeutigam, M. Ferm, M. Haas, M. Hangartner, P. Hofschreuder, A. Kasper-Giebl, H. Römmelt, J. Striedner, W. Terzer, L. Thöni, H. Werner and R. Zimmerling, J. Environ. Monit., 1999, 1, 259–265 RSC.
- L. Thöni, P. Brang, B. Rihm and E. Seitler, Environ. Monit. Assess. Search PubMed , submitted for publication.
- M. Kirchner, S. Braeutigam, H. Römmelt, E. Feicht and A. Kettrup, Gefahrstoffe – Reinhalt. Luft, 2000, 60, 383–388 Search PubMed.
- Ostluft Projekt: Verkehrs- und Schadstoffmessung 2002 im Gubrist Tunnel. E2 Controlling Verkehrsemissionen Tunnelmessungen, www.ostluft.ch.
- Th. R. Thijsse, J. H. Duyzer, H. L. M. Verhagen, G. P. Wyers, A. Wayers and J. J. Möls, Atmos. Environ., 1998, 32, 333–337 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
