Donor-substituted peralkynylated “radiaannulenes”: novel all-carbon macrocycles with an intense intramolecular charge-transfer†
Frieder
Mitzel
a,
Corinne
Boudon
b,
Jean-Paul
Gisselbrecht
b,
Paul
Seiler
a,
Maurice
Gross
b and
François
Diederich
*a
aLaboratorium für Organische Chemie, ETH-Hönggerberg, CH-8093 Zürich, Switzerland. E-mail: diederich@org.chem.ethz.ch
bLaboratoire d'Electrochimie et de Chimie Physique du Corps Solide, UMR 7512, C.N.R.S., Université Louis Pasteur, 4, rue Blaise Pascal, 67000 Strasbourg, France
First published on 9th June 2003
Abstract
A novel class of planar, highly conjugated all-carbon macrocycles, which we christened “radiaannulenes”, have been prepared based on acetylenic scaffolding using tetraethynylethene (TEE) building blocks; these structures are powerful electron acceptors and, upon peripheral substitution with electron-donating N,N-dialkylanilino groups, display intense intramolecular charge-transfer.
Recently, we reported perethynylated expanded radialenes1 and perethynylated dehydroannulenes2 bearing peripheral electron-donating N,N-dialkylanilino groups and showed that these compounds exhibit strong intramolecular charge-transfer absorptions. Here we present a novel class of mono- (1–3) and bi-cyclic (4, 5) expanded acetylenic chromophores which, from a structural viewpoint, are hybrids between perethynylated dehydroannulenes and expanded radialenes and which we therefore call perethynylated radiaannulenes.
The synthesis of 1–3 proceeded via the acyclic precursors 6–8 by intramolecular oxidative acetylene coupling (Scheme 1).‡ Compounds 6–8 in turn were assembled from the appropriate known mono- and cis-bis-deprotected tetraethynylethenes (TEEs),1–5 also by acetylene coupling (ESI).
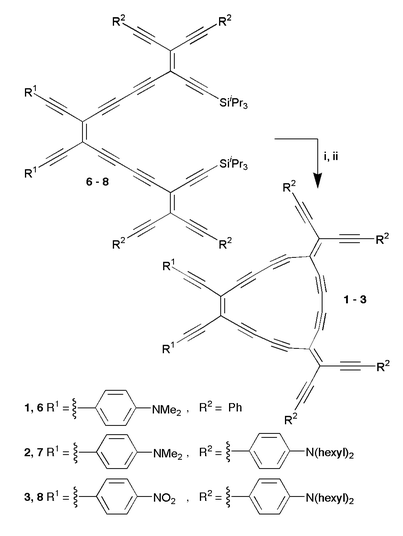 | ||
| Scheme 1 Synthesis of monocycles 1–3. Reagents and conditions: i, Bu4NF, THF, 0 °C, 10 min; ii, CuCl, N,N,N′,N′-tetramethylethylenediamine (TMEDA), O2, acetone, r.t., 2 h, 48% (1), 32% (2), 14% (3). | ||
Single crystals of 1, suitable for X-ray crystallography, were grown by slow diffusion of hexane into a chloroform solution.§The cyclic framework is virtually planar, with a mean out-of-plane deviation of 0.040 Å and a maximum deviation of 0.091 Å
(C(6))
(Fig. 1). The bond angles around the C(1)–C(16) double bond are all close to the ideal angle of 120°
(117.0–122.0°). Strain in the 16-membered ring is expressed mainly at the macrocyclic C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C(sp2) angles (C(4)–C(5)–C(6), C(6)–C(7)–C(8), C(9)–C(10)–C(11) and C(11)–C(12)–C(13)) with a bending from ideally 180° to approximately 163°.
C–C(sp2) angles (C(4)–C(5)–C(6), C(6)–C(7)–C(8), C(9)–C(10)–C(11) and C(11)–C(12)–C(13)) with a bending from ideally 180° to approximately 163°.
 | ||
| Fig. 1 ORTEP plot of 1 with arbitrary numbering. H-atoms are omitted for clarity. Atomic displacement parameters at 183 K are drawn at the 30% probability level. Selected bond angles (°): C(4)–C(5)–C(6) 163.18(18), C(5)–C(6)–C(7) 111.76(14), C(6)–C(7)–C(8) 164.86(18), C(9)–C(10)–C(11) 163.88(19), C(10)–C(11)–C(12) 111.18(15), C(11)–C(12)–C(13) 162.78(17). | ||
The synthesis of the bicyclic scaffolds 4 and 5 was achieved by double intramolecular oxidative coupling of the novel acyclic TEE-pentamers 9 and 10 (Scheme 2), after removal of the silyl-protecting groups. The acetylenic pentamers in turn were obtained from fully deprotected TEE (C10H4) and appropriate mono-deprotected TEEs by acetylenic coupling (ESI†).
 | ||
| Scheme 2 Synthesis of bicycles 4 and 5. Reagents and conditions: i, Bu4NF, THF, 0 °C, 10 min; ii, CuCl, TMEDA, O2, acetone/PhH, THF, r.t., 2 h, 88% (4), 15% (5). | ||
The new macrocyclic compounds presented here show several reversible, exceedingly low reduction potentials in cyclic voltammetry experiments (CH2Cl2 + 0.1 M Bu4NPF6; potentials vs. Fc/Fc+), which demonstrates their strong electron-accepting power (ESI†). For instance, the first reduction potential of 1 occurs at −1.19 V, compared to −1.96 V for tetrakis(trimethylsilyl)-protected tetraethynylethene.6 The introduction of four more electron-donating anilino-groups in 2 results in a more negative first reduction potential (−1.34 V) which is virtually identical to that of the recently reported, structurally related hexakis(N,N-dimethylanilino)-substituted peralkynylated dehydro[18]annulene (11, see ESI†) (−1.36 V in THF).2 The replacement of two anilino groups in 2 by nitrophenyl groups (3) shifts the first reduction potential anodically to −1.07 V. The bicyclic cores 4 and 5 display extremely low first reduction potentials at −0.81 and −0.98 V, respectively. In fact, the potential of 4 is significantly lower than the first reduction potential of buckminsterfullerene C60 (−1.02 V under comparable conditions),7 which is touted as a very good electron acceptor.
The electron-accepting power of the acetylenic cores in combination with the peripheral electron donor groups gives rise to intense intramolecular charge-transfer (CT) absorptions. The longest-wavelength absorption maximum of the hexa-anilino-substituted monocycle 2 appears at λmax = 615 nm (2.02 eV, ε = 99800 M−1 cm−1) (Fig. 2). Upon acidification of the solution with p-toluenesulfonic acid (PTSA) and protonation of the donor moieties, this band disappears; neutralisation with triethylamine regenerates the original spectrum (ESI†), which proves the CT-character of this absorption. Radiaannulene 1 also undergoes intramolecular CT, but the CT-band is weaker and only observed as a shoulder at 588 nm (2.11 eV) as a result of the smaller number of electron donor moieties. The introduction of two p-nitrophenyl moieties into 3 shifts the end-absorption bathochromically by approximately 100 nm (0.22 eV) compared to that of 2.
 | ||
| Fig. 2 Electronic absorption spectra of 2 (blue), 3 (green) and 5 (red) in pure CHCl3 and after addition of PTSA (2: yellow, 5: black). | ||
The bicyclic radiaannulene 5 displays an unusually strong CT-absorption with an end-absorption at approximately 850 nm (1.46 eV), the lowest-energy end-absorption known for any TEE-oligomers. Again, the CT-band of 5 can be reversibly removed and regenerated by acidification/neutralisation (Fig. S2, ESI†).
In summary, two-dimensional acetylenic scaffolding based on TEE-building blocks has been advanced to the preparation of radiaannulenes, an unprecedented class of perethynylated all-carbon macrocycles that are hybrids between expanded radialenes and dehydroannulenes. The new carbon sheets are powerful electron acceptors and, upon peripheral donor-substitution, exhibit strong intramolecular charge-transfer absorptions.
Support by the ETH Research Council and the German Fonds der Chemischen Industrie is gratefully acknowledged. We thank Prof. H. Hopf (Braunschweig) for useful discussions.
Notes and references
- M. B. Nielsen, M. Schreiber, Y. G. Baek, P. Seiler, S. Lecomte, C. Boudon, R. R. Tykwinski, J.-P. Gisselbrecht, V. Gramlich, P. J. Skinner, C. Bosshard, P. Günter, M. Gross and F. Diederich, Chem. Eur. J., 2001, 7, 3263 CrossRef CAS.
- F. Mitzel, C. Boudon, J.-P. Gisselbrecht, M. Gross and F. Diederich, Chem. Commun., 2002, 2318 RSC.
- For a review on carbon-rich acetylenic scaffolding, see: F. Diederich, Chem. Commun., 2001, 219 Search PubMed.
- J. Anthony, A. M. Boldi, Y. Rubin, M. Hobi, V. Gramlich, C. B. Knobler, P. Seiler and F. Diederich, Helv. Chim. Acta, 1995, 78, 13 CrossRef CAS.
- R. R. Tykwinski, M. Schreiber, R. P. Carlón, F. Diederich and V. Gramlich, Helv. Chim. Acta, 1996, 79, 2249 CrossRef CAS.
- C. Boudon, J.-P. Gisselbrecht, M. Gross, J. Anthony, A. M. Boldi, R. Faust, T. Lange, D. Philp, J.-D. van Loon and F. Diederich, J. Electroanal. Chem., 1995, 394, 187 CrossRef CAS.
- (a) D. Dubois, G. Moninot, W. Kutner, M. T. Jones and K. M. Kadish, J. Phys. Chem., 1992, 96, 7137 CrossRef CAS; (b) L. Echegoyen, F. Diederich and L. Echegoyen, in Fullerenes: Chemistry, Physics and Technology, eds. K. D. Kadish and R. S. Ruoff, Wiley-VCH, New York, 2000, p. 1 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: schemes describing the full synthesis of 1–5, preparation and full spectral characterisation of 4, complete electronic absorption spectra, complete electrochemical data. See http://www.rsc.org/suppdata/cc/b3/b304130a/ |
| ‡ All new compounds were fully characterised by IR, UV/Vis, 1H and 13C NMR, mass spectrometry and microanalysis or HR-MS. All mono- and bi-cyclic radiaannulenes are stable at room temperature in the air for months. |
§ Crystal data for 1 at 183 K for (C70H40N2, Mr
= 909.04): triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), Dc
= 1.167 g cm−3, Z
= 2, a
= 13.6643(5), b
= 14.0449(6), c
= 15.9105(8)
Å, α
= 66.12(1), β
= 68.03(1), γ
= 83.06(1)°, V
= 2587.6(2)
Å3. Bruker-Nonius Kappa-CCD diffractometer, Mo-Kα radiation, λ
= 0.7107 Å. A black single crystal with linear dimensions of ca. 0.3 × 0.07 × 0.05 mm was obtained by slow diffusion of hexane into a CHCl3 solution. Final R(F)
= 0.060, wR(F2)
= 0.129 for 690 parameters and 7437 reflections with I > 2σ(I) and 2.65 < θ < 27.49°
(corresponding R-values based on all 11698 reflections are 0.108 and 0.151 respectively). CCDC reference number 208124. See http://www.rsc.org/suppdata/cc/b3/b304130a/ for crystallographic data in CIF or other electronic format.
(no. 2), Dc
= 1.167 g cm−3, Z
= 2, a
= 13.6643(5), b
= 14.0449(6), c
= 15.9105(8)
Å, α
= 66.12(1), β
= 68.03(1), γ
= 83.06(1)°, V
= 2587.6(2)
Å3. Bruker-Nonius Kappa-CCD diffractometer, Mo-Kα radiation, λ
= 0.7107 Å. A black single crystal with linear dimensions of ca. 0.3 × 0.07 × 0.05 mm was obtained by slow diffusion of hexane into a CHCl3 solution. Final R(F)
= 0.060, wR(F2)
= 0.129 for 690 parameters and 7437 reflections with I > 2σ(I) and 2.65 < θ < 27.49°
(corresponding R-values based on all 11698 reflections are 0.108 and 0.151 respectively). CCDC reference number 208124. See http://www.rsc.org/suppdata/cc/b3/b304130a/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2003 |
