Improvement of the hydrothermal stability of SAPO-34
F. D. P.
Mees
*a,
L. R. M.
Martens
b,
M. J. G.
Janssen
b,
A. A.
Verberckmoes
b and
E. F.
Vansant
a
aUniversity of Antwerp (U.I.A.), Department of Chemistry, Universiteitsplein 1, B-2610, Wilrijk, Belgium. E-mail: Filip.Mees@ua.ac.be
bExxonMobil Chemical Europe Inc., Hermeslaan 2, B-1831 Machelen, Belgium
First published on 22nd November 2002
Abstract
Hydrothermal stability of SAPO-34 is greatly improved by the treatment of the acidic form of the SAPO-34 with NH3.
In 1982 the family of aluminosilicate zeolites was extended by the discovery of the aluminophosphate zeolites.1,2 In these crystalline microporous aluminophosphates (AlPO4’s), the tetrahedral (T) sites are occupied alternately by Al3+ and P5+ ions. By substituting a Si for a P a negatively charged framework is obtained so that Brønsted acidity can be introduced. Silicon substituted AlPO4’s are designated SAPO-n where n represents a structure type.3 SAPO-34, which is structurally analogous to the zeolitic mineral chabazite,4 is known to be a very powerful catalyst for the MTO-process (methanol-to-olefins), exhibiting high light olefin selectivities.5
SAPO-34 can be synthesised with either TEAOH (tetraethylammonium hydroxide) or morpholine as a template. It has been found for the morpholine templated SAPO-34 that, after activation, the catalyst’s structure and its acidity deteriorate rapidly and irreversibly during exposure to moisture.6 This rapid loss of acidity and structural integrity is a major drawback for their applicability. Since the rational design of superior catalysts is often based on post-synthesis modification techniques, there is a need for template-free, stabilised catalysts.
We report a new method for the stabilisation of hydrothermally unstable SAPO-34, based on the reversible reaction of NH3 with the acid sites.7 It will be shown that the deterioration of the framework and consequently the Brønsted acidity is caused by hydrolysis reactions starting at the acid sites. These hydrolysis reactions are essentially irreversible.6 After transforming the acidic form of the SAPO-34 into an NH4+-form, the reaction of H2O with acid sites is prevented. The interaction of H2O with other framework atoms appears to be much weaker and is reversible.
SAPO-34 was crystallised in the presence of morpholine as a templating agent, following a procedure described elsewhere.†7 The as-synthesised SAPO-34 was activated in a muffle furnace at 625 °C for 4 h (heating rate: 5 °C min−1) to form an H+-SAPO-34. Part of the activated material was transferred in a dynamic gas volumetric adsorption apparatus and reacted with NH3 at 220 °C leading to an NH4+-SAPO-34. The NH4+-SAPO-34 and the H+-SAPO-34 were transferred in stainless steel, Teflon lined autoclaves and steamed at 110 °C under autogeneous pressure for 5 to 25 h. The steamed samples were dehydrated in a muffle furnace at 625 °C (heating rate: 5 °C min−1) for 4 h and characterized together with the activated parent sample. During dehydration, NH3 is simultaneously desorbed from the NH4+-SAPO-34.
The steamed samples were thermogravimetrically analysed. The H+-SAPO-34 shows a strong weight loss at 140 °C due to desorption of water. The NH4+-SAPO-34 shows weight losses at 80 °C and at 400 °C attributed to desorption of physisorbed H2O and chemisorbed NH3, respectively. This shift from 140 °C to 80 °C in the DTG curve already indicates a strongly weakened interaction of the NH4+-SAPO-34 with water. The desorption of NH3 at 400 °C indicates that it is possible to regenerate strong acid sites (Brønsted acidity) after the steaming. This is confirmed by infrared spectroscopy (Fig. 1). After dehydration and removal of the NH3, the characteristic Brønsted bands at 3600 and 3625 cm−1 reappear for the NH4+-SAPO-34. The H+-SAPO-34 however lost a large part of the original acidity after only 5 hours of steaming and all of its acidity after 25 h of steaming. In the area of the framework vibrations between 500 and 900 cm−1, strong absorption bands are observed at 535, 573 and 645cm−1 for the fresh H+-SAPO-34 and the steamed, dehydrated NH4+-SAPO-34. These bands, however are absent in the spectra of the steamed, dehydrated H+-SAPO-34.
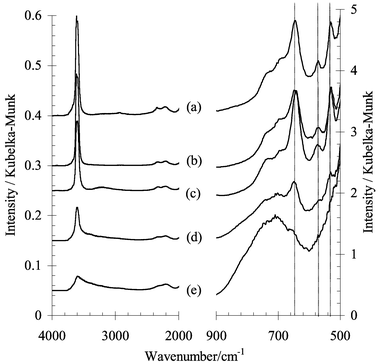 | ||
| Fig. 1 In situ diffuse reflectance IR spectra of samples (a) fresh H+-SAPO-34, (b) and (c) NH4+-SAPO-34 after 5 h and 25 h steaming respectively, (d) and (e) H+-SAPO-34 after 5 h and 25 h steaming respectively. The spectra were recorded at 400 °C under vacuum. Prior to recording the spectra, the samples were dehydrated and deammoniated at 625 °C. | ||
Not only the acidity of the fresh H+-SAPO-34 is protected by the transformation into an NH4+-SAPO-34, also the crystallinity is preserved during steaming as can be seen in Fig. 2. The X-ray diffractograms show that an unprotected H+-SAPO-34 rapidly loses its structure during steaming. The intensity of the diffraction peaks has diminished severely after only 5 h of steaming and a very broad band appears around 2θ = 22°. The NH4+-SAPO-34 however maintains its structural integrity, even after an extended period of steaming. The results from the infrared measurements together with the X-ray diffractograms strongly indicate that the deterioration of the SAPO-34 framework during steaming starts at the acid sites and is essentially irreversible.
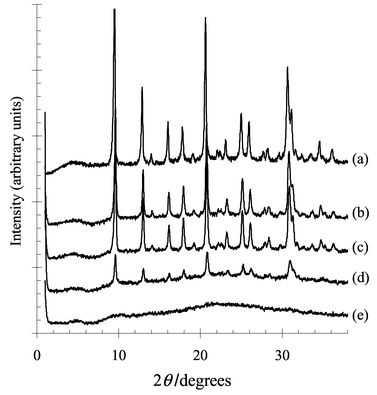 | ||
| Fig. 2 XR diffractograms of samples (a) fresh H+-SAPO-34, (b) and (c) NH4+-SAPO-34 after 5 h and 25 h steaming respectively, (d) and (e) H+-SAPO-34 after 5 h and 25 h steaming respectively. | ||
These findings are further supported by N2-adsorption and desorption measurements (Fig. 3). For the SAPO-34, typical Langmuir type I isotherms are observed. The isotherms of the fresh H+-SAPO-34 and of the steamed (25 h), dehydrated NH4+-SAPO-34 are essentially the same, while the steamed, dehydrated H+-SAPO-34 has a much lower N2-uptake. The micropore volume of the steamed, dehydrated NH4+-SAPO-34, as calculated from De Boer’s t-method, is the same as that of the parent sample (0.26 cc g−1). The unprotected H+-SAPO-34, however, lost almost all of its micropore volume after 25 h of steaming.
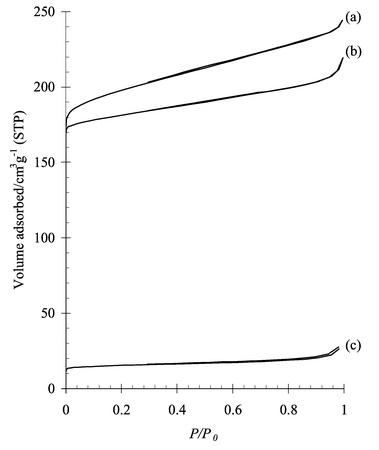 | ||
| Fig. 3 N2 sorption isotherms of (a) fresh H+-SAPO-34, (b) NH4+-SAPO-34 after 25 h steaming and (c) H+-SAPO-34 after 25 h of steaming. | ||
In summary, the results presented here strongly indicate that structural deterioration of H+-SAPO-34 during steaming starts on the Brønsted acid sites and is irreversible. Our method transforms the H+-SAPO-34 into an NH4+-SAPO-34 in a reversible way. It has been proven that the NH4+-SAPO-34 can withstand severe steaming for an extended period without loss of its structural integrity and acidity. This simple procedure to stabilise SAPO-34 is an important step forward to the commercial applicability of these materials.
Notes and references
- S. T. Wilson, B. M. Lok, C. A. Messina, T. R. Cannan and E. M. Flanigen, J. Am. Chem. Soc., 1982, 104, 1146 CrossRef CAS.
- S. T. Wilson, B. M. Lok and E. M. Flanigen, US Patent 4310440, 1982.
- B. M. Lok, C. A. Messina, R. L. Patton, R. T. Gajek, T. R. Cannon and E. M. Flanigen, US Patent 4440871, 1984.
- M. Ito, Y. Shimoyama and Y. Saito, Acta Crystallogr., 1985, C41, 1698 CAS.
- S. W. Kaiser, US Patent 4499327, 1983; S. W. Kaiser, US Patent 4677242, 1987; L. Juan, S. Q. Zhao, H. Y. Li, W. G. Guo and M. L. Ying, Stud. Surf. Sci. Catal., 1989, 46, 59 Search PubMed; M. W. Anderson, B. Sulikowski, P. J. Barrie and J. Klinowski, J.Phys. Chem., 1990, 94, 2730 Search PubMed; T. Inui, S. Phatanasri and H. J. Matsuda, J. Chem. Soc., Chem. Commun., 1990, 205 Search PubMed.
- M. J. G. Janssen, C. W. M. Van Oorschot, S. C. Fung, L. R. Martens, W. J. Mortier, R. G. Searle, M. M. Mertens, and S. N. Vaughn, US Patent 6316683, 2001.
- Patent pending.
Footnote |
| † Electronic supplementary information (ESI) available: detailed description of the synthesis procedure of SAPO-34. See http://www.rsc.org/suppdata/cc/b2/b210337k |
| This journal is © The Royal Society of Chemistry 2003 |
