Site-specific cleavage of human telomerase RNA using PNA-neocuproine·Zn(II) derivatives†
Andrew
Whitney
,
Gérald
Gavory
and
Shankar
Balasubramanian
*
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, UK CB2 1EW
First published on 26th November 2002
Abstract
Here we report the synthesis of a novel PNA based neocuproine·Zn RNA cleaving agent; we demonstrate that such agents sequence specifically cleave a synthetic RNA target and in particular the RNA component of human telomerase.
Sequence-specific cleavage of RNA using designed synthetic molecules is an important goal for chemists.1 Such agents have the potential to cleave complex RNA targets and serve as ribonuclease mimics for applications in therapeutics and molecular biology. The general features of ribonuclease mimics must incorporate a nucleic acid sequence recognition domain and functionalities that can mediate phosphate diester cleavage. A promising approach has been to covalently link a DNA oligonucleotide with a chemical cleaving head which may be metallated2 or metal-free.3 The most efficient metal-based cleaving systems have employed lanthanide metals and have demonstrated near quantitative cleavage of short synthetic RNA oligomers.4 Other strategies have employed synthetic DNA mimics as the target recognition component5 and more recently acridine derivatised oligonucleotides have been used as co-factors for free metal ion cleavage.6 Such studies have been initiated with simple synthetic RNA targets, and in some cases expanded to demonstrate cleavage of longer more complex targets, such as tRNA7 or the gag mRNA HIV transcript.2a
In this paper we report the design and synthesis of PNA-neocuproine–Zn(II) derivatives. These agents site-specifically cleave synthetic RNA and also the catalytic region of the RNA component of human telomerase.
We have chosen to base our system on the metal chelating agent 2,9-dimethyl-1,10-phenanthroline (neocuproine) whose complex with Zn(II) can promote the hydrolytic cleavage of RNA8 and thus serve as a biocompatible ribonuclease mimic.9 The PNA backbone has been employed owing to its improved target affinity, sequence specificity and biochemical stability.10 PNA synthesis is carried out with solid phase peptide coupling chemistry using Boc- or Fmoc-protected nucleobase derivatives of N-(2-aminoethylglycine).11Fig. 1 shows the route employed to prepare the neocuproine-bearing PNA monomer 1, in which neocuproine has been attached via a four-carbon spacer to the PNA backbone. 5′-Succinylaminoneocuproine 2 was coupled to amine 3 with EDC, to give orthogonally protected PNA monomer 4. Removal of the Boc group with 80% TFA/DCM yielded the desired compound 1.
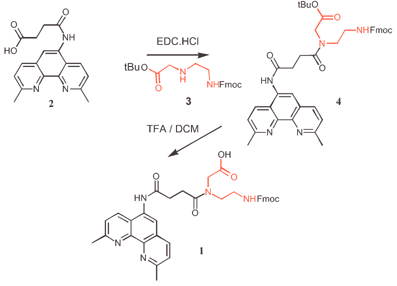 | ||
| Fig. 1 Synthesis of neocuproine-PNA monomer. | ||
We have chosen a biologically important and structurally complex target for this study: the RNA component of human telomerase (hTR).12 Telomerase is the reverse transcriptase enzyme responsible for synthesis and maintenance of telomeric repeats at the chromosome end.13 Telomerase function is essential to sustain the long-term replicative potential of human cells and the enzyme has become a therapeutic target for anticancer agents.14 Telomerase consists of a protein and an RNA component. Targeting selected regions of human telomerase RNA, either before15 or after complexation with the protein subunit, with oligonucleotide mimics such as PNA15 and PNA-derivatives,16 or more recently LNA17 has been shown to inhibit telomerase function. Ribozyme mediated cleavage of hTR has been reported to inhibit telomerase.18 We reasoned that hTR represents a challenging and important target for the study of site-specific cleavage by a synthetic ribonuclease mimic.
The catalytic region of hTR, an 11 nucleotide templating domain,17 which was the target for these studies (Fig. 2). Initial studies employed a 21 mer RNA (hTR21) corresponding to nucleotides 41 to 61 of hTR,11 containing the template domain (Fig. 2).
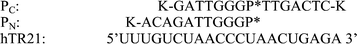 | ||
| Fig. 2 PNA PC and PN with P* indicating the position of neocuproine. Lysine has been end conjugated to aid solubility. | ||
Two distinct PNA cleavers (PC and PN) were prepared by inserting monomer 1 in the appropriate synthetic cycle (Fig. 2). Each cleaver was designed to cleave the target at the same site. PN positions the neocuproine at the N-terminus of the PNA, whereas in PC the neocuproine unit is at an internal site. The two alternatives were synthesized to evaluate the effect of allowing the cleaving head more (PN) or less (PC) conformational flexibility. A design such as PC could also ultimately lead to a cleaving system with product release and a catalytic mode of action. UV melting studies of PC and PN with hTR21 gave similar Tm values of 70 and 74 °C.
Fig. 3 shows a polyacrylamide gel of the RNA fragments generated from a series of cleavage experiments on 5′-32P labeled hTR21.19 Lanes 6 and 7 show site specific RNA cleavage by both PC and PN with a cleavage efficiency of 12 ± 2% and 30 ± 2%, respectively, after a 24 h incubation at 37 °C.20 The cleavage site is located between nucleotides C and U consistent with expectation (see arrow in Fig. 3). Lanes 1 and 2 confirm that hTR21 is stable to buffer and Zn(II) respectively. Lanes 3 and 4 show that PC and PN require the presence of Zn(II) to cleave RNA. The slightly reduced efficiency of PC relative to PN suggests that a more flexible linker to the neocuproine unit may be beneficial.
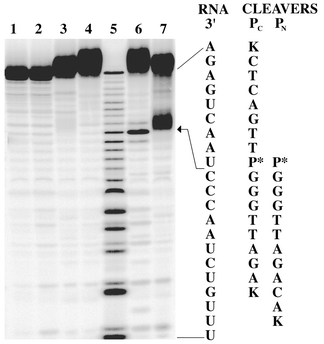 | ||
| Fig. 3 A 20% polyacrylamide gel showing hTR21 cleavage. With the exception of marker lane 5, all experiments were carried out in 10 mM HEPES/100 mM NaOCl4 buffer (pH 7.4) and contained 0.1 μM hTR21. Lane 1: control. Lane 2: +10 μM Zn(OAc)2. Lane 3: +5 μM PN, 50 μM EDTA. Lane 4: +5 μM PC, 50 μM EDTA. Lane 5: Alkaline hydrolysis marker lane. Lane 6: +5 μM PC, 10 μM Zn(OAc)2. Lane 7: +5 μM PN, 10 μM Zn(OAc)2. Reactions were carried out at 37 °C in a total volume of 20 μl for 24 h and quenched with EDTA loading buffer. | ||
To expand this study we evaluated the ability of PC and PN to cleave hTR. As part of the experimental design it was necessary to extend the natural 451 mer to a 647 mer.21 hTR was prepared by run-off transcription using T7 RNA polymerase in the presence of [α32P] UTP to incorporate the radiolabel. Fig. 4 shows a 4.5% polyacrylamide gel of RNA fragments generated from cleavage experiments with hTR. Lanes 1 and 4 show site-specific cleavage with PC and PN. The relative migration of the bands through the polyacrylamide gel is consistent with the expected fragment sizes of 399 and 248 nucleotides. It is interesting that the efficiency of hTR cleavage by PC was the same, within error, as for the short model oligonucleotide target, hTR21. Conversely, the efficiency of PN was reduced when going to the more complex target.22 The apparent advantage of a more flexible linker to the neocuproine moiety of PN for the model RNA, may well be compromised in the hTR target which is believed to have significant secondary structure.12
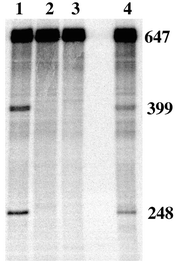 | ||
| Fig. 4 Site-specific cleavage of hTR. All experiments were carried out in 10 mM HEPES/100 mM NaOCl4 buffer (pH 7.4) and contained 0.01 μM hTR. Lane 1: +5 μM PC, 10 μM Zn(OAc)2. Lane 2: +10 μM Zn(OAc)2. Lane 3: Control. Lane 4: +5 μM PN, 10 μM Zn(OAc)2. Reactions were carried out at 37 °C in a total volume of 20 μl for 24 h and quenched with EDTA loading buffer. | ||
These studies provide the first demonstration of a PNA-based ribonuclease mimic that utilises a metal-cleaving system. The levels of RNA cleavage are as high as have been observed for a zinc-cleaving head.8 This paves the way for further developments using this therapeutically relevant and structurally complex RNA target.
We thank Drs Xiaohai Liu and Yamuna Krishnan-Ghosh for technical assistance and the BBSRC for a studentship. Details of synthesis and characterization of compounds is provided as ESI,† with the experimental detail of cleavage reactions.
Notes and references
- (a) B. N. Trawick, A. T. Daniher and J. K. Bashkin, Chem. Rev., 1998, 98, 939 CrossRef CAS; (b) M. Komiyama, J. Sumaoka, A. Kuzuya and Y. Yamamoto, Methods Enzymol., 2001, 341, 455–468 Search PubMed.
- (a) J. K. Bashkin, E. I. Frolova and U. Sampath, J. Am. Chem. Soc., 1994, 116, 5981–5982 CrossRef CAS; (b) J. Hall, D. Huesken and R. Haener, Nucleic Acids Res., 1996, 24, 3522–26 CrossRef CAS; (c) D. Magda, M. Wright, S. Crofts A. Lin and J. L. Sessler, J. Am. Chem. Soc., 1997, 119, 6947–6948 CrossRef CAS; (d) W. C. Putnam, A. T. Daniher, B. N. Trawick and J. K. Bashkin, Nucleic Acids Res., 2001, 29, 2199–2204 CrossRef CAS.
- (a) M. Komiyama and T. Inokawa, J. Biochem., 1994, 166, 719–720 Search PubMed; (b) M. A. Reynolds, T. A. Beck, P. B. Say, D. A. Schwartz, B. P. Dwyer, W. J. Daily, M. M. Vaghefi, M. D. Metzler, R. E. Klem and L. J. Arnold Jr., Nucleic Acids Res., 1996, 24, 760–5 CrossRef CAS; (c) V. Vlassov, T. Abramova, T. Godovikova, R. Giege and V. Silnikov, Antisense Nucleic Acid Drug Dev., 1997, 7, 39–42 CAS; (d) M. Endo, Y. Azuma, Y. Saga, A. Kuzuya, G. Kawai and M. Komiyama, J. Org. Chem., 1997, 62, 846–52 CrossRef CAS.
- J. Hall, D. Huesken, U. Pieles, H. E. Moser and R. Haener, Chem. Biol., 1994, 1, 185–190 CrossRef CAS.
- J. C. Verheijen, B. A. L. M. Dieman, E. Yeheskieky, G. A. van der Marel and J. H. van Boom, Angew. Chem., Int. Ed., 2000, 39, 369–372 CrossRef CAS.
- A. Kuzuya, R. Mizoguchi, F. Morisawa, K. Machida and M. Komiyama, J. Am. Chem. Soc., 2002, 124, 6887–6894 CrossRef CAS.
- N. G. Beloglazova, V. N. Silni’kov, M. A. Zenkova and V. V. Vlassov, FEBS Lett., 2000, 481, 277–280 CrossRef CAS.
- W. C. Putnam and J. K. Bashkin, Chem. Commun., 2000, 767–768 RSC.
- Zn was used in these experiments at 10 μM compared with the in vivo concentration of zinc in human serum of 17.2 μM.8.
- V. V. Demidov, V. N. Potaman, M. D. F. Kamenetskii, M. Egholm, O. Bushart, S. H. Sonnischsen and P. E. Nielsen, Biochem Pharmacol., 1994, 48, 1310–1313 CrossRef CAS.
- B. Hyrup and P. E. Nielsen, Bioorg. Med. Chem., 1996, 4, 5–23 CrossRef CAS.
- J. L. Chen, M. A. Blasco and C. W. Greider, Cell, 2000, 100, 503–514 CrossRef CAS.
- G. W. Greider and E. H. Blackburn, Cell, 1985, 42, 405 CrossRef CAS.
- N. W. Kim, Eur. J. Cancer, 1997, 33, 781 CrossRef CAS.
- S. E. Hamilton, C. G. Simmons, I. S. Kathiriya and D. R. Corey, Chem. Biol., 1999, 6, 343–351 CrossRef CAS.
- J. G. Harrison, C. Frier, R. Laurant, R. Dennis, K. D. Raney and S. Balasubramanian, Bioorg. Med. Chem. Lett., 1999, 9, 1273–1278 CrossRef CAS.
- A. N. Elayadi, D. A. Braash and D. R. Corey, Biochemistry, 2002, 41, 9973–9981 CrossRef CAS.
- Y. Yokoyama, Y. Takahashi, A. Shinohara, Z. L. Lian, X. Y. Wan, K. Niwa and T. Tamaya, Cancer Res., 1998, 58, 5406–5410 Search PubMed.
- The high stability of the initial PNA/RNA duplex is apparent with an RNA band shift visible for the full length RNA in lanes from reactions bearing either PNA oligomer. This is also the case for the duplex product formed by cleavage mediated by oligomer PN.
- Cleavage efficiency based on three independent experiments.
- In order to better resolve the cleavage products from starting material using electrophoresis.
- Cleavage efficiency based on two independent experiments.
Footnote |
| † Electronic supplementary information (ESI) available: experimental section. See http://www.rsc.org/suppdata/cc/b2/b210135a/ |
| This journal is © The Royal Society of Chemistry 2003 |
