First example of a taxane-derived propellane in Taxus canadensis needles
Qing Wen
Shi
a,
Françoise
Sauriol
b,
Orval
Mamer
c and
Lolita O.
Zamir
*a
aHuman Health Research Center, INRS-Institut Armand-Frappier, Université du Quebec, 531 Boulevard des Prairies, Laval, Quebec, Canada H7V 1B7
bDepartment of Chemistry, Queen′s University, Kingston, Canada K7L 3N6
cBiomedical Mass Spectrometry Unit, McGill University, 1130 Pine Avenue west, Montreal, Quebec, Canada H3A 1A3
First published on 28th November 2002
Abstract
The first example of a propellane isolated from the needles of a yew is reported; the biogenesis from a putative taxane precursor is proposed.
Tricyclic saturated hydrocarbons systematically named tricyclo[a.b.c]alkanes have been referred to as [a.b.c]propellanes. These structures attracted the imagination of synthetic organic chemists as early as 1972.1 On the other hand, the first [3.3.3]-propellane natural product isolated was the sesquiterpenoid carbocyclic compound: modhephene obtained from the roots of Liabum eggersii or the leaves and stems of the toxic plant Isocoma wrightii.2
In this publication, we report the second [3.3.3]natural product propellane 1 (Fig. 1) which was isolated from the needles of the yew, Taxus canadensis. This 2α,9α,10β-triacetoxy-5α-hydroxy-3,11-cyclo-12,20-cyclotaxa-13-one 1 is here given the trivial name Canataxapropellane which is based on plant origin and carbon skeleton. The structure was identified by High Resolution Fast Atom Bombardment Mass Spectrometry (HRFABMS) and 2D NMR spectroscopy. The relative stereochemistry of 1 (Fig. 2) was defined using nuclear Overhauser enhancement and exchange spectroscopy (NOESY).
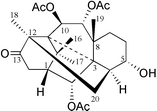 | ||
| Fig. 1 The structure of taxane 1. | ||
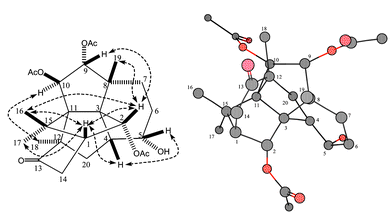 | ||
| Fig. 2 Relative stereochemistry of 1: the figure on the left shows the NOESY correlations in dotted arrows and the one on the right the three dimensional calculated structure MM2. | ||
The molecular composition of 1,3 C26H36O8, was established from both HRFABMS m/z 477.2488 [M+H]+ and 13C NMR spectroscopic data. The 1H NMR spectrum of 1 revealed four proton signals corresponding to the four methyl groups at δ 1.09, 1.12, 1.22 and 1.58; two of them were geminal methyl groups as shown by a COSY correlation (long range W-type coupling). Three acetyl groups were assigned (methyls at δ 2.01, 2.02, 2.08 and carbonyls at δ 169.7, 169.7, 170.7). These signals, together with its Taxus origin, suggested that 1 was a taxane derivative.4 Analysis of the 1H and 13C NMR, and HMQC spectra revealed that 1 contains three acetyls, one ketone, four oxymethines, two methines, four methylenes, five quaternary carbons and four methyl groups. Interestingly, no signal was observed in the olefinic region of the 13C NMR spectrum, suggesting that the C-11–C-12-double bond, found in most taxanes, was saturated in 1. This observation was confirmed by the chemical shifts of methyl-18 (δ 1.22, 3H, s) and of H-10 (δ 5.46, 1H, d) in the 1H NMR and by the chemical shift of the C-13 ketone at δ 216.9 in the 13C NMR spectrum. These data are very different from those of taxanes with a C-11–C-12-double bond where the H-18 chemical shift is in the range of δ 2.0–2.1 and H-10 at δ 6.1–6.4 and C-13 carbonyl at 200–205.4 Additionally, the characteristic proton signals due to an exocyclic methylene group or to the geminal protons of a C-4(20)-epoxide or a C-4,5-oxetane ring fused to ring C were absent in the 1H and 13C NMR spectra.4 The connectivities of the protons on the taxane skeleton of 1 were determined by analysis of the 1H–1H COSY spectrum. Interpretation of 1H and 13C NMR, and HMBC spectra permitted the positional assignment of the functional groups and the quaternary carbons. The 1H NMR spectrum of 1 showed signals for four protons attached to oxygenated carbons (three acetate groups and one free hydroxyl group). An AB doublet of doublets resonating at δ 5.57 and δ 5.46 with a large coupling constant of J = 9.8 Hz attributed to H-9 and H-10 suggested that two acetyl groups were attached to C-9 and C-10,4 respectively. These assignments were confirmed by the correlations of H-9 and H-10 with the carbonyl signals at δ 170.7 and 169.7 in the HMBC spectrum. Methyl-18 correlated with C-20 and H-20 with the ketone at C-13 in the HMBC spectrum, indicating a connection between C-20 and C-12 to form a new five membered ring in 1. The signals resonating at δ 2.61 and 2.53 with a large geminal coupling constant of 20.2 Hz, correlated to C-13 and C-2 in the HMBC spectrum, were assigned to H-14a and H-14b, respectively. Using H-14b as a starting point, the signals of H-1 and H-2 were confirmed by the 1H–1H COSY spectrum. The chemical shift of H-2 at δ 5.71 (1H, d, J = 5.2 Hz) suggested the presence of an acetyl group on C-2 as verified by the HMBC correlations. The signal observed at δ 4.12 was assigned to H-5, and this indicates the presence of a free hydroxyl on C-5. The spin system from H-4→H-5→H-6→H-7 was easily interpreted using H-5 as a starting point in the 1H–1H COSY spectrum. The cross-peaks of H3-16 and H3-17 to C-1, C-11 and C-15 indicated that Me-16 and Me-17 were connected to C-15, while the 1H–13C long-range correlations of H3-19 to C-3, C-7, C-8 and C-9 were indicative of the presence of Me-19 at C-8. The remaining two quaternary carbons at δ 63.6 and δ 62.7, which were correlated to H-1, H-10, Me-16, Me-17 and Me-19, H-2 and H-4, respectively, were attributed to C-11 and C-3. The H-3 proton, which usually resonates as a doublet at δ 2.5–3.8 in most taxanes was absent.4 Since one unsaturation equivalent was left, no olefinic carbon signal was observed in the 13C NMR spectrum and C-3 and C-11 are the only quaternary carbons left, we can only suggest that C-3 and C-11 are connected to form one new ring as shown in 1. The C-3 resonance at δ 62.7 (instead of δ 38–43 in regular taxanes)4 supports this assignment. The structure of 1 was, therefore, 2α,9α,10β-triacetoxy-5α-hydroxy-3,11-cyclo-12,20-cyclotaxa-13-one (Fig. 1) which we named Canataxapropellane.
The relative stereochemistry of 1 (Fig. 2) was defined on the basis of the nuclear Overhauser enhancement and exchange spectroscopy (NOESY) data, chemical shifts and their coupling constants. The magnitudes of the vicinal coupling constant (J = 9.8 Hz) between H-9 and H-10 indicated that H-9 and H-10 were configured in a trans-orientation as in other taxanes. The H-2 proton displayed strong NOE correlations with Me-16, Me-19 and H-1; this NOE pattern is similar to that observed in paclitaxel derivatives where H-2, Me-16, Me-19 and H-1 are on the β-side of the molecule. The β-orientation of H-5 was supported by the observations of strong NOESY correlations of H-5/H-4, and H-5/H-14a. The α-orientation of H-10 was confirmed by the NOESY correlations of H-10/H-18 and H-10/H-6a. Me-18 interacts with H-10 and H-20b suggesting that it has an α-configuration as in other C-12 substituted 3,11-cyclic taxanes.4
A putative biogenesis of 1 is proposed in Scheme 1 with taxinine A, previously isolated from the needles of the Canadian yew,5 as a starting material. The first step would be a rearrangement of the 4(20) double bond to a more stable 3,4-tetra-substituted compound that we named 20-deoxytaxezopidine B, by analogy with taxezopidine B with a C-20-hydroxyl group which has been found in the seeds of the Japanese yew.6 Abstraction of an allylic hydrogen from C-20 would lead to a 3,11-cyclic intermediate with an enol-form on C-12–C-13. The three dimensional structure of taxanes assures the proximity of the C-4–C-20 and C-12–C-13 double bonds, causing the last cyclisation to form a bond between C-20 and C-12 in Canataxapropellane 1.
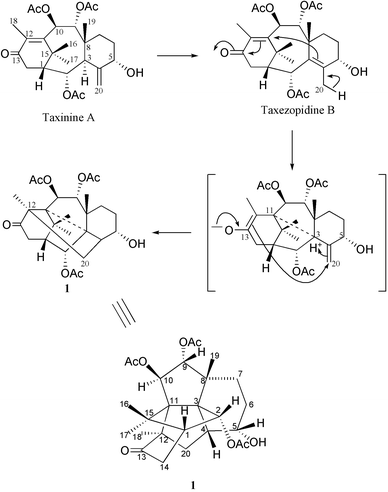 | ||
| Scheme 1 Proposed mechanism for the formation of 1. | ||
We thank the Natural Science and Engineering Research Council of Canada and the Canadian Breast Cancer Research Initiative for support via an operating grant to L. O. Z. We thank the INRS-Institut Armand-Frappier for a postdoctoral fellowship to Q. W. S.
Notes and references
- Syntheses of propellanes (a) B. Fuchs, Y. Auerbach and M. Sprecher, Tetrahedron Lett., 1972, 2267 CrossRef CAS; (b) C. A. Dvorak and V. H. Rawal, J. Chem. Soc., Chem.Commun., 1997, 2381 RSC and references therein.
- Isolation of [3.3.3] propellanes (a) L. H. Zalkow, R. N. Harris and D. Van Derveer, J. Chem. Soc., Chem. Commun., 1978, 420 RSC; (b) F. Bohlmann, C. Zdero, R. Bohlmann, R. M. King and H. Robinson, Phytochemistry, 1980, 19, 579 CrossRef CAS.
- Canataxapropellane 1 [α]D22 +11° (c 0.25, CHCl3). 1H NMR (500 MHz, CDCl3): 2.21 (1H, br.t, J = ∼6.1 Hz, H-1); 5.71 (1H, d, J = 5.2 Hz, H-2); 2.55 (1H, o.m, H-4); 4.12 (1H, m, H-5); 2.00 (1H, o.m, H-6a); 1.46 (1H, m, H-6b); 2.00 (1H, o.m, H-7a); 1.54 (1H, o.m, H-7b); 5.57 (1H, d, J = 9.8 Hz, H-9); 5.46 (1H, d, J = 9.8 Hz, H-10); 2.61 (1H, d, J = 20.2 Hz, H-14a); 2.53 (1H, dd, J = 20.2, 7.2 Hz, H-14b); 1.12 (3H, s, Me-16); 1.58 (3H, s, Me-17); 1.22 (3H, s, Me-18); 1.09 (3H, s, Me-19); 1.97 (1H, o.m, H-20a); 1.74 (1H, o.m, H-20b); 2.08 (3H, s, CH3CO–); 2.02 (3H, s, CH3CO–); 2.01 (3H, s, CH3CO–). 13C NMR (125 MHz, CDCl3): 47.0 (C-1); 76.9 (C-2); 62.7 (C-3); 43.8 (C-4); 65.1 (C-5); 24.4 (C-6); 28.0 (C-7); 38.6 (C-8); 82.0 (C-9); 76.3 (C-10); 63.6 (C-11); 60.8 (C-12); 216.9 (C-13); 38.9 (C-14); 42.2 (C-15); 24.3 (C-16); 28.3 (C-17); 18.7 (C-18); 20.6 (C-19), 42.6 (C-20); 20-21 (3 × CH3CO–); 169.7 (CH3CO–); 170.7 (CH3CO–); 169.7 (CH3CO–). HMBC correlations (CDCl3, H/C): 1/2, 1/11, 1/13, 1/15; 2/1, 2/3, 2/4, 2/8 or 2/14, 2/169.7; 4/2, 4/3, 4/5, 4/6, 4/8, 4/20; 9/7, 9/8, 9/10, 9/19, 9/170.7, 10/9, 10/11, 10/12, 10/15, 10/169.7; 14a/1, 14a/2, 14a/14; 14b/1, 14b/2, 14b/13; 16/1, 16/11, 16/15, 16/17; 17/1, 17/11, 17/15, 17/16; 18/11, 18/12, 18/13, 18/20; 19/3, 19/7, 19/8, 19/9; 20a/13; 20b/13. NOESY correlations (CDCl3, H/H): 1/2, 1/14b, 1/16, 1/17, 1/20a; 2/1, 2/17, 2/19; 4/1, 4/5, 4/18; 5/4; 6a/5, 6a/19; 7a/5, 7a/19; 7b/7a, 7b/19; 9/2, 9/17, 9/19; 10/6a, 10/7a, 10/20a, 10/18; 14a/14b; 14b/1, 14b/5, 14b/14a, 14b/18; 20b/18, 20b/20a. HRFABMS m/z 477.2488 [M + H]+ (calc. for C26H37O8, 477.2488).
- (a) D. G. I. Kingston, A. A. Molinero and J. M. Rimoldi, in Progress in the Chemistry of Organic Natural Products, ed. W. Herz, G. W. Kirby, R. E. Moore, W. Steglich and C. H. Tamm, Springer Verlag, New York, 1993, 61, 1–206; Search PubMed; (b) G. Appendino, in The Chemistry and Pharmacology of Taxol and Its Derivatives, ed. V. Farina, Elsevier, Amsterdam 1995, 22, 1–53 and 55–101 Search PubMed.
- L. O. Zamir, J. Zhang, J. Wu, F. Sauriol and O. Mamer, Tetrahedron, 1999, 55, 14323 CrossRef CAS.
- X. Wang, H. Shigemori and J. Kobayashi, J. Nat. Prod., 1998, 61, 474 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
