Efficient degradation of organic pollutants mediated by immobilized iron tetrasulfophthalocyanine under visible light irradiation
Xia
Tao
a,
Wanhong
Ma
a,
Jing
Li
a,
Yingping
Huang
a,
Jincai
Zhao
*a and
Jimmy C.
Yu
b
aLaboratory of Photochemistry, Center for Molecular Science, Institute of Chemistry, The Chinese Academy of Science, Beijing, 100080, China. E-mail: jczhao@infoc3.icas.ac.cn; Fax: +86-10-62559373; Tel: +86-10-82616495
bDepartment of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China
First published on 27th November 2002
Abstract
Supported iron tetrasulfophthalocyanine can efficiently catalyze the degradation of organic pollutants by H2O2 under visible light irradiation in an aqueous solution, and the catalyst can be easily recycled without apparent loss of activity.
Soluble metalloporphyrins and metallophthalocyanines have attracted much attention for their application in oxidative degradation of organic pollutants in water in the presence of an oxygen atom donor.1 A new trend of this field is to develop supported complexes for facile recovery and easy separation of the catalyst.2 It has been reported that iron tetrasulfophthalocyanine (FePcS) immobilized on ion-exchange resin could effectively catalyze the oxidation of polychlorophenols by H2O2 in a CH3CN–H2O solvent mixture .3 However, the conversion rate of the organic compounds was greatly reduced if water was used as the sole solvent. The requirement of an organic co-solvent seriously limits its application in water treatment. Based on the visible light absorption property of FePcS, we have recently demonstrated the degradation of organic pollutants in aqueous solutions of FePcS and H2O2 without addition of any organic solvents.4 In this photoassisted reaction, HO˙ radicals have been observed to be the main reactive species for the degradation of organics.
Here we report the development of a photocatalytic treatment system that consists of immobilized FePcS under visible light irradiation for the degradation of organic pollutants by H2O2. In this photoassisted process, water is used as the sole solvent. A poly(vinylbenzene) resin (Amberlite-IRA 900) is used as the support for FePcS. The ammonium groups in the resin bind to the sulfonato groups of FePcS by electrostatic interactions resulting in a stable heterogeneous photocatalyst .5Fig. 1 presents the time profile for the degradation of Orange II, one of the main pollutants from textile and photographic industries, in water under different conditions. Under light irradiation with a 500-W halogen lamp (light filter λ <450 nm), the complete disappearance of Orange II in the aqueous FePcS-resin/H2O2 solution was observed after photoreaction for only 50 min. In control experiments under irradiation with visible light, Orange II was scarcely degraded after 60 min of photoreaction in the heterogeneous solutions containing only resin (curve a) or the resin loaded by FePcS (curve b). In the presence of both FePcS-resin and H2O2, no significant degradation of Orange II was observed in the dark after 60 min (curve c). The absorbance decreased by ca. 30% (from point A to B) before light irradiation. This was attributed to the dark adsorption of Orange II on the resin surface. Besides, the photodegradation of Orange II was also evident from the color change on the catalyst surface. Before irradiation, the catalyst was brown, due to the adsorbed dye of Orange II. The solution became colorless after exposure to visible light for 50 min (curve d in Fig. 1). Further irradiation for 20 min changes the catalyst to blue (inherent colour of support FePcS), indicating that Orange II both on the surface of support catalyst and in bulk solution can be efficiently degraded. However, in the other three cases (curves a–c in Fig. 1), the color of resin or FePcS-resin in the Orange II solution remained orange or brown even if the reaction time exceeded 140 min.
 | ||
| Fig. 1 Degradation of Orange II (0.2 mM, 50 ml) under different conditions; (a) in the presence of resin (10 mg) only under irradiation with visible light; (b) in the presence of FePcS-resin (10 mg) under irradiation with visible light; (c) in the presence of FePcS-resin (10 mg) and H2O2 (5 mM) in the dark; (d) under otherwise identical conditions as (c) except irradiation with visible light. Molar ratio of FePcS/Orange II is 0.014∶1. | ||
Since the FePcS-resin catalyst can be easily recovered by filtration, we carried out three consecutive degradations of Orange II with FePcS-resin as the photocatalyst. The FePcS-resin was repetitively utilized each time after the color of Orange II both in bulk solution and on the catalyst surface disappeared. Fig. 2 confirms that the activity of the photocatalyst can be maintained. During the photoreaction process, no FePcS was found in the bulk solution, as measured by both UV/Vis spectroscopy and HPLC. The results indicate that the supported FePcS is an efficient and rather stable photocatalyst for the degradation of organic pollutants in water under visible light irradiation.
 | ||
| Fig. 2 Supported FePcS-resin catalyst recycling in repetitive degradation of Orange II (0.2 mM, 10 ml) by H2O2 (5 mM) in the presence of FePcS-resin (10 mg). | ||
A remarkable feature is the enhanced photocatalytic efficiency of FePcS after immobilizing onto a resin (TON >14). The catalytic activity of FePcS-resin in this heterogeneous system (molar ratio of FePcS/substrate = 0.014–0.068∶1) is remarkably enhanced compared to that in homogeneous system (molar ratio of FePcS/substrate >1∶3).4 A reasonable explanation for the activity enhancement of the immobilized FePcS is that the phthalocyanine (Pc) rings of FePcS in solution are essentially planar and stacked in a parallel fashion along one of the axes.6 The interaction of FePcS with resin may block the stack of FePcS to a certain extent, which then allows H2O2 to easily approach the iron center of FePcS at the axial direction to give [HOOFePcS]. This results in the activation of H2O2.7,8 Under visible light irradiation, [HOOFePcS] can be excited and then undergoes rapid cleavage to generate HO.radicals that can degrade organic pollutants effectively.
The degree of the substrate mineralization (the initial system contained 0.2 mM of Orange II, 5 mM of H2O2 and 10 mg of FePcS-resin in which molar ratio of FePcS/Orange II is 1.36%) was evaluated by determination of the total organic carbon (TOC). The TOC removal yield was 25% after the suspension was irradiated continuously for 250 min. When the degradation products were analysed by gas chromatography–mass spectrometry (GC-MS), five different peaks appeared in the chromatogram, which correspond to formic acid 1, N,N-dimethylformamide 2, oxalic acid 3, 1,2-benzenedicarboxylic acid 4 and 1,2-benzenediformic anhydride 5, respectively (Table 1). These products of mainly small organic acids show low toxicity and are all biodegradable.1a,9 In control experiments, no signals of the products from the Orange II degradation were observed for the reaction carried out in the absence of H2O2 under the same conditions, or in the presence of FePcS-resin and H2O2 in the dark.
|
Peak
no. |
Retention
time/min |
Identified intermediates by MS |
Structural
formula |
|---|---|---|---|
| a The samples for GC–MS (Trio-2000, column PEG20M, size 30 M × 0.25 mm) in the photodegradation of Orange II (0.2 mM) in the presence of FePcS-resin (10 mg) and H2O2 (5 mM) under visible light irradiation were prepared as follows: the degraded solution was first filtered through a Millipore filter (pore size 0.22 μm), vaporized directly (below 323 K) under reduced pressure, and the residue was then dissolved in methanol. See text for details. | |||
| 1 | 6.53 | Formic acid | HCOOH |
| 2 | 11.20 | N,N-Dimethylformamide | HCON(CH3)2 |
| 3 | 12.08 | Oxalic acid | HOOC–COOH |
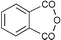
| 4 | 23.69 | 1,2-Benzenedicarboxylic acid |

|
| 5 | 26.09 | 1,2-Benzenediformic anhydride |
|
Furthermore, we also carried out the degradation of Sulforhodamine B (SRB) and salicylic acid (SA) in the presence of FePcS-resin/H2O2 under visible light irradiation and found that both SRB and SA could be easily degraded. These results demonstrate the advantages of the immobilized FePcS over the unsupported FePcS for effective elimination of organic pollutants in water utilizing visible light or direct sunlight. This new approach might be applicable as an alternative or complementary method for the treatment of organic pollutants in wastewater.
This work was supported financially by NSFC (Nos. 20133010, 20077027, 20277038 and 4001161947), CAS, China National Committee for Science and Technology, and Research Grants Council of the Hong Kong Special Administrative Region (Project No. N-CUHK 433/00).
Notes and references
- (a) S. S. Cupta, M. Stadler, C. A. Noser, A. Ghosh, B. Steinhoff, D. Lenoir, C. P. Horwitz, K. W. Schramm and T. J. Collins, Science, 2002, 296, 326–328 CrossRef; (b) G. Labat, J.-L. Seris and B. Meunier, Angew. Chem., 1990, 102, 1488–1450; G. Labat, J.-L. Seris and B. Meunier, Angew. Chem., Int. Ed. Engl., 1990, 29, 1471–1473 CrossRef; (c) A. Sorokin, J.-L. Séris and B. Meunier, Science, 1995, 268, 1163–1165 CAS.
- (a) A. Sanjuan, M. Alvaro, G. Aguirre, H. Garcia and J. C. Scaiano, J. Am. Chem. Soc., 1998, 120, 7351–7352 CrossRef CAS; (b) B. Meunier, Chem. Rev., 1992, 92, 1411–1456 CrossRef CAS; (c) G. Labat and B. Meunier, J. Org. Chem., 1989, 54, 5008–5011 CrossRef CAS.
- A. Sorokin and B. Meunier, J. Chem. Soc., Chem. Commun., 1994, 1799–1800 RSC.
- (a) X. Tao, W. Ma, T. Zhang and J. Zhao, Angew. Chem., 2001, 113, 3103–3105 CrossRef; X. Tao, W. Ma, T. Zhang and J. Zhao, Angew. Chem., Int. Ed., 2001, 40, 3014–3016 CrossRef CAS; (b) X. Tao, W. Ma, T. Zhang and J. Zhao, Chem. Eur. J., 2002, 8, 1321–1326 CrossRef CAS.
- FePcS-resin was prepared by adding 0.5 g of Amberlite-IRA 900 (from Aldrich Chemical Co.) to an aqueous solution of 10 mg of FePcS. After 24 h of gentle stirring, the material was filtered off and washed with water. In the course of the loading, the concentration of FePcS in the aqueous solution was monitored by UV/Vis spectroscopy. Afterwards, the material was dried at room temperature, and then at 65 °C for 60 h. FePcS-resin catalyst exhibits a strong blue colour. Amount of FePcS loaded 13 mg/1 g resin.
- Z. Z. Ho, C. Y. Ju and W. M. Hetherington III, J. Appl. Phys., 1987, 62, 716–718 CrossRef CAS.
- DPD method was used for determination of H2O2 in the Orange II photodegradation in the presence of only soluble FePcS or FePcS-resin, respectively. The results show that the decomposition of H2O2 is much faster in the heterogeneous system (ΔC/Δt = 5.0 × 10−5 mol L−1 min−1) than that in the homogeneous (ΔC/Δt = 2.1 × 10−6 mol L−1 min−1).
- (a) Y. Saito, M. Mifune, S. Nakashima, J. Odo and Y. Tanaka, Talanta, 1987, 34, 667–669 CrossRef CAS; (b) J. Odo and H. Hashimoto, Anal. Sci., 1998, 14, 935–939 Search PubMed.
- J. Fernandez, J. Bandara, A. Lopez, Ph. Buffat and J. Kiwi, Langmuir, 1999, 15, 185–192 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
