Low molecular weight organogelators for water
Guijun
Wang
and
Andrew D.
Hamilton
*
Department of Chemistry, Yale University, New Haven, CT 06520, USA. E-mail: andrew.hamilton@yale.edu; Fax: 203 432 3221; Tel: 203432 5570
First published on 8th January 2003
Abstract
Mono-urea serine derivatives with low molecular weights were prepared in one step and show remarkable self-assembling and gelation properties in water.
Of the several classes of organogelators that have been discovered in recent years, the majority form gels in organic solvents.1 In contrast, certain polymers are well known to form gels in water and the resultant material is called a hydrogel. Hydrogels have a range of biomedical applications, in such areas as tissue engineering, controlled release drug delivery systems, and medical implants.2 While it is relatively easy to understand the properties of a polymer that lead to swelling within a hydrogel, the physical counterparts by which small molecules might self-assemble and thicken water are not so well understood. A key reason for this is the paucity of small molecules able to gel pure water. There are only a few examples of low molecular weight organogelators that are able to gel water or even water mixtures with other solvents. To date the only molecular based self-assembly that results in water gelation includes bis-urea carboxylate derivatives,3 several sugar-based gelators,4–8 tartrate-based gemini surfactants,9 amino acid derivatives10 (that function in water/DMSO mixtures), a bile acid derivative that gels aqueous acids,11 bis-oxalyl amides12 and more recently, positively charged L-lysine derivatives.13 In this paper we report a new class of simple urea derivatives that are not only easy to prepare but also have the smallest molecular weight of any known water gelator.
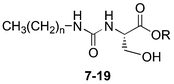
We had earlier shown that a series of serine-based long chain bis-urea derivatives are efficient gelators of organic solvents.14 Modification of these derivatives may offer a potential route to hydrogelators. Compounds 1–6 are bis-urea derivatives containing a shorter chain serine ester as the exterior alkylamine component. The presence of a hydroxyl group offers the potential for additional hydrogen bonding interactions beyond those formed between the urea groups. Compounds 1–6 were shown to form gels in water in the presence of other polar organic solvents such as THF, ethanol, acetone and DMSO. In the course of searching for small molecule gelators that can gel pure water, we sought to modify the structures of these molecules to improve both their solubility and their gelation ability. This was achieved by simply cutting the molecules in half, removing one urea head group and leaving mono-urea serine derivatives 7–19 (Table 1). The free hydroxyl group can interact with water and improve solubility while the short alkyl chain may inhibit crystallization and lead to gelation. We have synthesized a series of these mono-urea derivatives and shown them to be effective gelators of water.
| Compound | n | R | MW | Statea | Cb | Mc |
|---|---|---|---|---|---|---|
| a G, gel at room temperature, S: soluble in water (>0.1 g ml−1), P: precipitation, I: insoluble when heating, G/P the mixture formed unstable gel which precipitates. b C, concentration of the gel in wt%. c M, concentration in mol L−1. | ||||||
| 7 | 3 | Me | 218 | S | ||
| 8 | 4 | Me | 232 | G | 8 | 0.34 |
| 9 | 5 | Me | 246 | G | 1.5 | 0.061 |
| 10 | 7 | Me | 274 | G/P | ||
| 11 | 3 | Et | 232 | S | ||
| 12 | 4 | Et | 246 | G | 1.5 | 0.061 |
| 13 | 5 | Et | 260 | G | 0.8 | 0.031 |
| 14 | 7 | Et | 288 | P | ||
| 15 | 11 | Et | 358 | I | ||
| 16 | 2 | Bn | 280 | G | 1.0 | 0.036 |
| 17 | 3 | Bn | 294 | G | 0.8 | 0.027 |
| 18 | 4 | Bn | 308 | G/P | ||
| 19 | 5 | Bn | 322 | I | ||
The water gelating properties of the mono-urea serine derivatives are shown in Table 1. Methyl, ethyl and benzyl ester derivatives of L-serine were studied and compared with different alkylamide substituents on the other side of the urea functional group. The optimal alkylurea chain lengths were butyl for benzyl ester, pentyl for ethyl ester, and hexyl for methyl ester. In all cases, gelation was achieved at concentrations below 1.5 wt%. From these results we can predict other structural variants that should lead to good water gelators. For non-branching aliphatic side chains, the optimal total chain length of both the serine ester and the alkylurea should be six to nine. For branched side chains, a further two to three methylene groups should be tolerated. For aromatic esters, shorter chain lengths in the alkylurea are expected to give optimal gelation properties. The molecular weights of 8, 9, 12 are below 250 Da and so they represent the smallest water gelators reported to date.
In order to study the influence of different aromatic groups on gelation, we synthesized several benzyl ester analogs 20–24. Their gelation properties are shown in Table 2. The aromatic ureas showed decreased aqueous solubility compared to the aliphatic derivatives, however 22–24 exhibited good gelation properties in water. Compounds 25–27 containing polymerizable methacrylate groups were also prepared. The serine methyl ester and ethyl ester derivatives 25 and 26 are soluble in water. The benzyl derivative 27 is an excellent hydrogelator, and can gel water at concentrations as low as 1.0 wt%. From these results, we can predict that the behaviour of the methacryloyl group resembles that of a propyl group. The branched and carboxylate ester groups increase the solubility of the ureas in water. Increasing the chain length of aliphatic R2 will lead to a decrease in solubility and effective gelation in water.
| Compound | R1 | R2 | Statea | Cb |
|---|---|---|---|---|
| a G, gel at room temperature, P, precipitation, S, soluble. b C. Concentration of the gel in wt%. | ||||
| 20 | Bn | Me | G | 2.5 |
| 21 | Bn | Et | G | 3.3 |
| 22 | Bn | Bn | G | 2.0 |
| 23 | 4-Bromobenzyl | Et | G | 0.5 |
| 24 | 4-Bromobenzyl | Bn | G | 1.0 |
| 25 | Methacryloxyethyl | Me | S | |
| 26 | Methacryloxyethyl | Et | S | |
| 27 | Methacryloxyethyl | Bn | G | 1.0 |
By scanning electronic microscopy, the hydrogels showed fibrous aggregation features (Fig. 1). Hexylurea 9 is an efficient gelator, with gels in water appearing to be composed of intertwined fibrous networks (Fig. 1a). Gelator 12 has the same molecular weight as 9 and formed strongly aggregated clusters of small fibers. The analogous hexylurea 13 formed similar types of fibrous structures as 9 (Fig. 1b) while benzyl esters 16 and 17 form aggregated planar sheets and fibers, with the compacted fibrous character of the sheets indicating some local crystallinity (Fig. 1c). We also investigated the morphology of those compounds that were not good water gelators. The octylurea methyl ester 10 was not very soluble in water, but formed unstable gels composed of straight fibers. The higher analog of benzyl derivatives 18 and 19 exhibited closed packed aggregation structures.
 | ||
| Fig. 1 SEM of the gelators in water a–g: corresponding to 9; 13; 17; 23; 24; 27, 20. h: polarized optical micrograph of 20. | ||
The gelators containing aromatic groups showed better gelation properties than the aliphatic ureas probably due to π–π stacking5 and some of them gave interesting self-assembling morphologies. Methyl ester 20 formed isolated long and straight fibers (Fig. 1g) that are also birefringent (Fig. 1h). Ethyl ester 21 is soluble in hot water but formed a gel at higher concentrations upon cooling. The benzyl ester 22 did not completely dissolve on heating but still formed a stable non-homogeneous gel composed of short fibers and strips. In contrast, the ethyl and benzyl esters of 4-bromobenzylurea 23 and 24 both readily formed gels in water. The ethyl derivative 23 formed an entangled curved fibrous network (Fig. 1d), while the benzyl ester formed more straight fibers (Fig. 1e). Methacrylate derivatized 27 also showed a fibrous type of aggregate (Fig. 1f) with a diameter larger than in the other cases.
In summary, we have reported a family of small amino acid derivatives that can form gels in water without any co-solvents or additives. The hydrogelators reported here have the lowest molecular weights among non polymeric hydrogelators.
We thank the National Science Foundation (CHE 9817240) for financial support of this work.
Notes and references
- Reviews on organogelators: P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133 Search PubMed; D. J. Abdallah and R. G. Weiss, Adv. Mater., 2000, 12, 1237 CrossRef.
- Reviews on hydrogels and applications: K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869 Search PubMed; T. Miyata, T. Uragami and K. Nakamae, Adv. Drug Delivery Rev., 2002, 54, 79 CrossRef.
- L. A. Estroff and A. D. Hamilton, Angew. Chem., Int. Ed., 2000, 39, 3447 CrossRef CAS.
- J. H. Jung, G. John, M. Masuda, K. Yoshida, S. Shinkai and T. Shimizu, Langmuir, 2001, 17, 7229 CrossRef CAS.
- O. Gronwald and S. Shinkai, J. Chem. Soc., Perkin Trans. 2, 2001, 1933–1937 RSC; O. Gronwald and S. Shinkai, Chem. Eur. J., 2001, 7, 4328 CrossRef CAS.
- R. Luboradzki, O. Gronwald, M. Ikeda, S. Shinkai and D. N. Reinhoudt, Tetrahedron, 2000, 56, 9595 CrossRef CAS.
- S. Bhattacharya and S. N. G. Acharya, Chem. Mater., 1999, 11, 3504 CrossRef CAS.
- H. Kobayashi, A. Friggeri, K. Koumoto, M. Amaike, S. Shinkai and D. N. Reinhoudt, Org. Lett., 2002, 4, 1423 CrossRef CAS.
- R. Oda, I. Huc and S. J. Candau, Angew. Chem., Int. Ed., 1998, 37, 2689 CrossRef CAS.
- F. M. Menger and K. L. Caran, J. Am. Chem. Soc., 2000, 122, 11679 CrossRef.
- U. Maitra, S. Mukhopadhyay, A. Sarkar, P. Rao and S. S. Indi, Angew. Chem., Int. Ed., 2001, 40, 2281 CrossRef CAS.
- M. Jokic, J. Makarevic, M. Zinic and J. Makarevic, Chem. Commun., 1995, 1723 RSC; M. Jokic, B. Peric, V. Tomisic, B. Kojic-Prodic and M. Zinic, Chem. Eur. J., 2001, 7, 3328 CrossRef CAS.
- M. Suzuki, M. Yumoto, M. Kimura, H. Shirai and K. Hanabusa, Chem. Commun., 2002, 884 RSC.
- G. Wang and A. D. Hamilton, Chem. Eur. J., 2002, 8, 1954 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |