D. Jed Harrison, University of Alberta†
Abstract
The Analystprofiles Professor Jed Harrison
 Jed Harrison was born in 1954 in Vancouver, Canada. He received his BSc in Chemical Physics from Simon Fraser University in 1980, and received the Gordon Shrum Gold Medal upon graduation. He performed graduate studies in Chemistry at the Massachusetts Institute of Technology under the supervision of Professor Mark Wrighton and graduated with a PhD in 1984. He later joined the Chemistry Department at the University of Alberta in 1984, and was promoted to Full Professor in 1994. He was the 1993 recipient of the Canadian Society for Chemistry’s W.A.E. McBryde Medal, awarded for research in Analytical Chemistry. In 1994 he received the University of Alberta’s first Faculty of Science Research Award. In 1996 he received the Steacie Memorial Fellowship, one of the Natural Science and Engineering Council of Canada’s most prestigious awards. He was awarded the 1996 Heinrich Emanuel Merck Prize, which he shares with Prof. Andreas Manz. He has recently shared the 2003 Golay award with Andreas Manz and Michael Ramsey, and will be presented the Canadian Society for Chemistry’s 2003 Maxxam Award for Analytical Chemistry, in addition to being elected to Royal Society of Canada’ Academy of Science. His research interests are in the application of micromachining to ‘lab-on-a-chip’ technologies and biochemical analysis.When did you first become interested in science and for what reason?
Jed Harrison was born in 1954 in Vancouver, Canada. He received his BSc in Chemical Physics from Simon Fraser University in 1980, and received the Gordon Shrum Gold Medal upon graduation. He performed graduate studies in Chemistry at the Massachusetts Institute of Technology under the supervision of Professor Mark Wrighton and graduated with a PhD in 1984. He later joined the Chemistry Department at the University of Alberta in 1984, and was promoted to Full Professor in 1994. He was the 1993 recipient of the Canadian Society for Chemistry’s W.A.E. McBryde Medal, awarded for research in Analytical Chemistry. In 1994 he received the University of Alberta’s first Faculty of Science Research Award. In 1996 he received the Steacie Memorial Fellowship, one of the Natural Science and Engineering Council of Canada’s most prestigious awards. He was awarded the 1996 Heinrich Emanuel Merck Prize, which he shares with Prof. Andreas Manz. He has recently shared the 2003 Golay award with Andreas Manz and Michael Ramsey, and will be presented the Canadian Society for Chemistry’s 2003 Maxxam Award for Analytical Chemistry, in addition to being elected to Royal Society of Canada’ Academy of Science. His research interests are in the application of micromachining to ‘lab-on-a-chip’ technologies and biochemical analysis.When did you first become interested in science and for what reason?Growing up during the ‘60s Space Race’ sparked a strong interest in astronomy and this was when I first contemplated becoming a scientist. After brief interludes as a warehouse man and playing in a rock and roll band I went to Simon Fraser University. Because of my interest in astronomy I wanted to major in physics but I was better at chemistry and so I decided to major in both. One Professor at University, an inorganic chemist named Roland Pomeroy (who trained at Imperial College, London) was particularly enthusiastic and I signed up to do research with him one summer. After that I decided I wanted to become a Professor in Chemistry. Also, at the time, there were only around 2000 jobs in the world for astronomers!
What are your perceptions of the health and vibrancy of research in analytical science today?In North America, analytical chemistry is a healthy field that has changed a lot in the last decade. There are some constants, e.g., separation science remains important but spectroscopy is not as highly a practised research area as it should be in academic research. In past times, there was a focus on atomic spectroscopy, but in the current climate of increased interest in biological problems and the life sciences in general, molecular spectroscopy is the key tool. Complex molecular spectroscopic methods like two-photon and confocal fluorescence microscopy are examples of techniques that need further development, e.g., new dye systems, development of instrumentation for higher sample throughput etc.
What qualities, in your opinion, are most important for becoming a successful scientist/analytical chemist these days?There are the obvious ones of clear thinking, ability to question our observations, good scientific judgement, good communication skills so that we can convey our ideas and what excites us to others. One thing I learned as a student at MIT is that you can select an important practical problem and do excellent science—you do not need to investigate esoteric concepts as if you live in an ivory tower. The challenge is to find an appropriate practical problem. In synthetic chemistry you can select a problem within your field—for analytical chemistry you need to design technology for people in other fields to use to solve their problems.
How did you get into the field of miniaturisation?I did my graduate studies at MIT on photoelectrochemical cells and so got a good background in semiconductors and electrochemistry from that work. When I started as a Professor at Alberta, with my electrochemical background, I decided to work on integrated chemical sensors, e.g., integrated glucose sensors and ISFETs. Although the sensors arena was a hot topic at that time, no sensor designed since the 1960s had been developed commercially so I started thinking about why this was the case. I came to the conclusion that the problem lay in the fact that sensors offer what amounts to only a single separation step, whereas in chromatography there are many thousands of separation steps and because of this the latter offers much more selectivity. So I decided to miniaturise separation steps and design devices in a sensor-like format. At that time (1987) the first electric motors on a chip (MEMS) were presented. Inspired, I wanted to think of ways to use that technology with what I was doing. In 1989 I met Andreas Manz at a conference in Switzerland and arranged a sabbatical with him at his laboratory at Ciba Geigy where we did the first lab-on-a-chip experiments (the CE chip).
 Tell us something about your current research projects?
Tell us something about your current research projects?We focus on lab-on-a-chip systems for protein preparation for proteomics. One of our main challenges is how to get the sample preparation into a chip format for the ease of the laboratory scientist, the ultimate goal being chips that prepare samples for detection by mass spectrometry.
We are also interested in multiplexing technology—increasing the number of channels on a chip is a big challenge. Also, processing and manipulating cells on a chip is a huge opportunity: developing tools for biologists to work with cells and allowing them to perform experiments they have not had the opportunity to do in the past. We are also beginning to ask the question whether ‘nano’ can have a role in the future, e.g., we have some exciting ideas utilising sub-cellular components as machines in nanosystems and using microfluidics to control sub-micron pores for biological systems.
 Which of your previous research are you most proud of?
Which of your previous research are you most proud of?Some work I carried out in the early 1990s on ion sensors and why they drift. This involved trying to understand polymer science and engineering different experimental setups to study how and why the sensor drifts. Also, the first CE experiment on a chip was an exciting moment as was the first system we designed that offered mixing, injection and separation (published in Science in 1993).
What scientific discovery would you like to have been responsible for and why?It would have been great to participate in the development of the scanning tunnel microscope and related scanning probe methods. These are powerful new tools that have revolutionized our ability to probe systems at the submicron level, and they continue to develop in exciting new ways that will lead to further new discoveries about the nature and behaviour of matter in the nano-scale.
Which trends in the miniaturisation community are you pleased about?The obvious one is how widely electrophoresis on a chip has been accepted and adopted: so many people make it work; companies in Japan, US and elsewhere are now selling products. One thing that is a shame, however, is that the commercial devices are all based on early/first discoveries in this field and that newer technology has not been exploited commercially yet. It is a shame because this is where the technology will really start to display advantages: integrating the different parts of an analysis. One of the reasons for this is that putting together the components of a total analytical system is much more difficult than we initially anticipated. Groups in this area have (understandably) focussed on the easy things first. What must be borne in mind is that this is a new technology which required a complete shift in thinking: new tools needed to be developed for shrinking systems and it has taken us 10 years to discover these tools in order to really progress.
 Are there any such trends that concern you?
Are there any such trends that concern you?Yes, people who think that you have to integrate everything on a chip. The fact is; you’re not supposed to.
How would you convert a lab-on-a-chip sceptic?A lot of criticism is based on false expectation. I think initial expectations of lab-on-a-chip were too high. People think that if you are designing a microfluidic system you are supposed to integrate it fully and if you do not achieve this then you have failed. This is not true. You need to find out which components need to be miniaturised and focus only on those. My feeling is that microfluidics is somewhat analogous to inventing the printed circuit board technology rather than the transistor—the technology will become ubiquitous but in a way that people will not notice—we would not have computers without it but no one celebrates the discovery of the printed circuit board!
For more on research in the Harrison group visit http://www.chem.ualberta.ca/faculty/harrison.htm
Footnote |
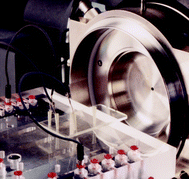
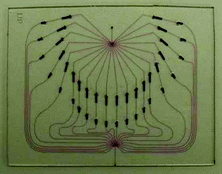
| † Photographs show Professor Harrison and a recent device designed by the Harrison group for chip mass spectrometry including the MS set-up, the chip in the holder and the chip alone with the channels filled with potassium permanganate. |
| This journal is © The Royal Society of Chemistry 2003 |
