Gallic acid: prospects and molecular mechanisms of its anticancer activity
A. P. Subramanian,
A. A. John,
M. V. Vellayappan,
A. Balaji,
S. K. Jaganathan*,
Eko Supriyanto and
Mustafa Yusof
IJN-UTM Cardiovascular Engineering Centre, Faculty of Bioscience and Medical Engineering, Universiti Teknologi Malaysia, Johor Bahru 81310, Malaysia. E-mail: jaganathaniitkgp@gmail.com; Fax: +60-7-5558553; Tel: +60-7-5558548
First published on 27th March 2015
Abstract
Cancer is the second leading cause of death worldwide. There is always a huge demand for novel anticancer drugs and scientists explore various natural and artificial compounds to overcome this. Gallic acid (GA) is one of the phenolic acids found in many dietary substances and herbs used in ancient medicine. It possesses antiinflammatory, antioxidant, antiviral and antibacterial properties. The present review summarizes the anticancer activity of GA and its derivatives. Various in vitro and in vivo experiments of GA against a variety of cancer cell lines were reported. The previous studies show that the anticancer activity of GA is related to the induction of apoptosis through different mechanisms like generation of reactive oxygen species (ROS), regulation of apoptotic and anti-apoptotic proteins, suppression and promotion of oncogenes, inhibition of matrix metalloproteinases (MMPs) and cell cycle arrest depending upon the type of cancer investigated. Conclusively, GA and its derivatives may be considered as a potent drug for cancer treatment alone as well as in combination with other anticancer drugs to increase the efficiency of chemotherapy. However, there is still a need for more experimentation in knock-out animal models and human clinical trials to promote and place GA and its derivatives on the commercial market.
1. Introduction
Cancer is the second major cause of death worldwide,1 and is a class of diseases characterized by uncontrolled cell proliferation. In the year 2014 alone, it is estimated that about 585![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 Americans will die from cancer, corresponding to about 1600 deaths per day.2 According to the survey of the world health organization, global cancer rates could increase by 50% in the year 2020, which is approximately to 15 million. Cancers of the lung and bronchus, prostate, and colorectal continue to be the most common causes of cancer death.3
720 Americans will die from cancer, corresponding to about 1600 deaths per day.2 According to the survey of the world health organization, global cancer rates could increase by 50% in the year 2020, which is approximately to 15 million. Cancers of the lung and bronchus, prostate, and colorectal continue to be the most common causes of cancer death.3
There are countless choices of treatments for cancer, with the prime ones including surgery, chemotherapy and radiotherapy.4 The selection of treatment depends upon the type, location and stage of the cancer as well as the person's health and wishes. Chemotherapy is one of the standardized treatments that employ chemotherapeutic agents to kill cells that divide rapidly.5 Chemotherapeutic drugs induce apoptosis, which is a programmed cell death involving biochemical events leading to morphological and molecular changes leading to death, in the cancer cells. Epirubicin, cisplatin, 5-fluorouracil, doxorubicin and cyclophosphamide are some of the anticancer drugs available in the market.6 Although there are plenty of drugs which can retard the cancer, it cannot cure cancer completely when detected at latter stages. Hence, there is a prolonged search for novel anticancer drugs. In addition to the synthetic drugs, scientists also explore the natural compounds from our diets.
Gallic acid (GA) is a phenolic acid found in many dietary substances. It is natural compound found in gallnuts, sumac, witch hazel (Hamamelis virginiana), clove (Syzygium aromaticum), tealeaves, oak bark, sundew and other plants. Edibles like blackberry, hot chocolate, common walnut, Indian gooseberry, vinegar, wine, white tea contain GA. Some of the sources of GA have been pictured in the Fig. 1. GA has also been reported to inhibit several cancer cell lines through multitude of mechanisms. However there is no single review encompassing the overall molecular actions through which gallic acid exert anticancer phenomenon. The current review shed the light on the anticancer activity and mechanism of cell death induced by GA and thereby promoting it as a plausible anticancer drug in the near future.
2. Source and chemistry of gallic acid
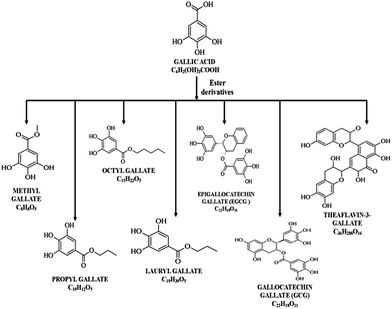
GA is a trihydroxybenzoic acid, a type of organic acid, also known as 3,4,5-trihydroxybenzoic acid. The chemical formula is C6H2(OH)3COOH. It is white, yellowish-white, or pale fawn-colored crystals soluble in alcohol, ether, glycerol and acetone. It is an organic acid found in a variety of foods and herbs, which are well known as powerful antioxidants. Many of the foods containing GA have been used for years as natural remedies. Blueberries,7 for example, were used by native Americans and the early American settlers to make aromatic tea that was used as a relaxant during childbirth and also as a good tonic for purifying the blood. The hazel balm and tea rich in GA were applied to cuts and wounds to prevent infection, and the tea alone was used to treat menstrual problems, colds and other illnesses.8 Chinese herbalists used gallnuts from oak and sumac to treat intestinal disorders, bleeding, hematochezia and hyperhidrosis.9 Thirty ayurvedic herbs and formulations have been screened for the presence of GA, which is already in use for treatments of different diseases over years.10GA can be produced by hydrolysis of tannic acid with acid or alkali or microbial tannase. GA is easily freed from gallotannins by oxidation. The most expedient method is to precipitate it from an aqueous solution using concentrated sulfuric acid. A slower means of obtaining the GA is to allow atmospheric oxygen to oxidize passively in water. It is mainly used for the synthesis of antibacterial drugs like trimethoprim in the pharmaceutical industry. In the food industry, GA is used as substrate for the chemical synthesis of food preservatives such as pyrogallol and gallates.11 The ester derivatives of GA are frequently identified as gallates in many plants and also investigated for their biological property. Structure of some of the ester derivatives are given in the Fig. 2. Researches related to the anticancer property of the alkyl esters have been reported. These studies indicated that gallates induced apoptosis in various cancer cell lines.56,57,61,65,75 It was found that the alkyl esters were more effective in inhibiting the cancer cell lines compared to GA. For example, lauryl gallate was found to be 40 times more potent than GA when experimented with mouse B cell lymphoma Wehi 231.30 This effect may be attributed to the hydrophobic moieties present in the gallates. Availability of more than eight carbons present in the alkyl ester increase the affinity to the cancer cell membrane and makes the drugs more permeable, thereby rendering the cancer cells more prone to the alkyl esters.12
3. Biological properties of gallic acid
The initial medicinal property reported was the antimalarial activity of GA.13 Later on, the antifungal activity of GA was demonstrated. In the same year, antibacterial activity of synthetic derivatives of GA against Bacillus subtilis, Staphylococcus aureus and Escherichia coli was studied.14 The antiviral activity of GA was demonstrated by the in vivo and in vitro experiments on the mortality of monkeys exposed to influenza.15 All these important findings allowed establishment of diverse and important activities of GA thereby opening a new scenario for this important natural compound.GA possesses good antioxidant activity, which is exerted through increase in the DNA damage and release of cytochrome c. It also decreased the glutathione and mitochondria potential of the cells.16 It also has a dose dependent antifungal property.17 It is used to treat albuminuria, diabetes and as a remote astringent in cases of internal haemorrhage. GA was found to show cytotoxicity against cancer cells, without inhibiting the healthy cells.18 Many alkyl derivatives of GA also possess anticancer properties, of which lauryl gallate, propyl gallate, epigallocatechin gallate (EGCG) and theaflavin-3-gallate are notable.19 In this scenario, this paper focuses on the anticancer property of GA and its derivatives against a range of cancer cell lines. GA also has good anti-inflammatory property. It has been reported that GA inhibited the activation of NF-κB-dependent p65 acetylation and production of inflammatory markers. The low acetylation rate of p65 resulted in a complete loss of function of NF-κB promoting GA as a new anti-inflammatory drug.20
4. Bioavailability and toxicity of gallic acid
Prior to introducing an anticancer drug in chemotherapy, the effects of drug apart from its anticancer property should also be studied. Bioavailability and toxicity are major factors among them. The term bioavailability refers to the investigation of the amount of a particular compound that is absorbed and available for physiological function, mostly in in vivo condition. This investigation also helps in finding the metabolites of the compound after absorption. The study of bioavailability and toxicity of the compound are the key factor before standardizing a chemotherapeutic drug.21 Bioavailability of GA has been investigated in both animal models and human trails. The O-methylation resulting in the formation of 4-O-methyl gallic acid (4OMGA) accounts for the major metabolite in the urine of rats or rabbits ingesting gallic acid, propyl gallate, lauryl gallate, or tannic acid.22 The intestinal absorption of GA in rats by oral administration was investigated by Konishi et al.23 The rats were given 100 μmol L−1 body weight of GA. GA was slowly absorbed and 0.71 μmol L−1 of GA and it's metabolite 4OMGA was found in serum. Shahrzad et al. determined GA and its metabolites in human plasma and urine by oral consumption of 50 mg of acidum gallicum tablets. The experimental results showed the presence of 4OMGA along with unchanged GA in the biological fluids like plasma and urine.24 This was followed by another study to evaluate the GA pharmacokinetics and bioavailability in healthy humans. The individuals were given acidumgallicum tablets (10% GA and 90% glucose) or black brew tea (0.3 mmol GA). After the consumption of acidum gallicum tablets, about 36.4 ± 4.5% unchanged GA and its metabolite 4OMGA in urine was found whereas it was 39.6 ± 5.1% for black tea. The bioavailability of GA from tea was estimated as 1.06 ± 0.26 comparing both tablet and tea consumption, depicting that the bioavailability of GA was independent of matrix of distribution.25 This report suggests that GA may be administered orally in the form of tablet or free form during chemotherapy. The bioavailability and efficacy of antioxidants like GA, quercetin, epigallocatechingallate (EGCG) and n-propyl gallate in human corneal limbal epithelial (HCLE) cells were measured to verify whether antioxidants might be beneficial constituents of lubricant eye drops. The ROS generation was reduced significantly when an antioxidant was present both in the medium with the xanthine oxidase and within the cells. This indicated that they are bioavailable and might be effective in protecting the corneal epithelium from oxidative damage.26Toxicity refers to the effect of a compound on the whole organism, another important factor to be remembered before consumption of a drug. The toxicity of GA has been investigated in mouse model and the no observed-adverse-effect level (NOAEL) was also determined. Rajalakshmi et al., experimented the administration of various doses in the mice model. The highest dose of 5000 mg kg−1 administered orally did not show any significant changes in the hematological parameters and said to be the NOAEL.27 Subchronic toxicity of GA was inspected in F344 rats by feeding diet containing 0, 0.2, 0.6, 1.7 and 5% GA for 13 weeks. Toxicological parameters such as clinical signs, body weight, food consumption, hematology, blood biochemistry, organ weights and histopathological assessment were made. There was gain in the body weight with 5% GA-treated animals of both sexes from week 1 to the end of the experiment. Toxic effects following administration of 0.6% or more in males and 5% in females resulted in reduction of haemoglobin concentration, hematocrit and red blood cell counts and increase in reticulocytes. Histopathological observation showed development of hemolytic anemia. In addition, centrilobular liver cell hypertrophy, reflected in increase in liver weight, was observed from 1.7%. Based on these toxicology data, 0.2% was determined to be a NOAEL in rats. This level was translated into 119 and 128 mg per kg per day, respectively for male and female rats.28
Similarly, the toxicity of propyl, octyl and dodecyl esters of GA have been examined widely in animal models involving oral administration. This study showed that the biokinetics of propyl gallate was different from octyl and dodecyl gallate which was due to the degree of absorption and hydrolysis. Liver enzyme induction was observed at 5000 mg kg−1 feed of propyl gallate. In contrast, the octyl gallate or dodecyl gallate showed affects at a dosage of 3000 mg kg−1 feed or higher levels. In summary, the FAO/WHO Joint Expert Committee on Food Additives (JECFA) accepted 1000 mg kg−1 feed as no-effect level and 0.2 mg kg−1 body weight (as a sum of propyl, octyl and dodecyl gallates) as an acceptable daily intake (ADI) for men.29
5. Anticancer activity of gallic acid and its derivatives
GA and its derivatives were found to be potent anticancer agent. There have been many literatures explaining the anticancer activity of GA and some of its derivatives against prostate cancer, oral cancer, melanoma, leukemia, lymphoma, colon cancer and breast cancer cells. The prominent molecular actions initiated by GA and its derivatives against the above mentioned cancer cells were enumerated in successive subtitles.5.1 Anticancer activity on lung cancer cells
Ohno et al. investigated the apoptosis-inducing effect of GA in four human lung cancer cell lines, small cell carcinoma (SBC-3), squamous cell carcinoma (EBC-1), adenocarcinoma (A549) and cisplatin-resistant sub-clone of SBC-3.30 GA had a dose dependent effect on the cancer cells. There was change in cell morphology, DNA fragmentation and loss of viability after GA treatment (IC50: 10, 20, 60 μg mL−1 correspondingly for the cell lines). This was continued by the study of in vivo anti-tumor effects of orally administered gallic acid on C57 black mice with transplanted LL-2 cells.31 The cells were treated with GA and/or cisplatin. The tumor weight of the mice treated with the combination of cisplatin and GA (IC50: 200 μM) was reduced compared to cisplatin alone. This recommends the combination of GA with an anti-cancer drug, as an effective protocol for lung cancer therapy. GA induced apoptosis in a dose-dependent manner with DNA fragmentation and changes in cell morphology. The apoptotic process also showed involvement of caspase activation and oxidative processes. These findings also suggest the possibility of GA in lung cancer therapy, especially to circumvent resistance to anti-cancer drugs. GA had an anti-cancer effect on Calu-6 and A549 lung cancer cells in relation to reactive oxygen species (ROS) and glutathione (GSH). The cell growth decreased in a dose-dependent (IC50: 10–50 μM & 100–200 μM respectively) way accompanied by the loss of mitochondrial membrane potential. GA-induced lung cancer cell death was related to GSH depletion as well as ROS level changes.32GA was found to induce a reactive oxygen species-provoked c-Jun NH2-terminal kinase-dependent apoptosis in lung fibroblasts cells.33 There was activation of c-Jun NH2-terminal kinase (JNK) in the mouse lung fibroblast cells treated with GA (IC50: 50 g mL−1). The initiator of JNK signaling pathways was found to be GA mediated hydrogen peroxide formation, followed by the activation of p53 pathway leading to apoptosis. Maurya et al. studied the anticancer property of GA in human lung adenocarcinoma cell line A459 and possible mechanisms related to GA induced.34 GA stimulated morphological changes like cell shrinkage and rounding up of the cells. The GA treatment also decreased mitochondrial membrane potential and increased intracellular reactive oxygen species activating the caspase-3. In contrast, the caspase-8 was not activated indicating the involvement of intrinsic pathway of cell apoptosis. The apoptosis induced by GA was in dose- and time-dependent manner.
5.2 Anticancer activity on prostate cancer cells
GA, the major anticancer compound suppressed the growth of DU145 prostate cancer cells. The reduction in the cell viability of DU145 cells involves generation of ROS and mitochondria-mediated apoptosis. GA caused the cell cycle arrest at the G2/M phases by the activation of Chk1 and Chk2 and inactivation of Cdc25C and Cdc2. Moreover, GA was found to have synergistic effect with doxorubicin in suppressing the proliferation of DU145 cells.35 The autoxidation of GA killed the malignant prostrate cells effectively. This autoxidation also produced notable increase of ROS level. There was loss of mitochondrial potential along with the release of cytochrome c leading to the activation of caspases-3, -8 and -9. GA induced a dose dependent apoptosis in prostate cancer cells.36Liu et al. tested the anticancer property of GA on PC3 prostate cancer cells.37 The percentage of viable cells after the treatment of GA was found to reduce in a time and dose-dependent (50, 100, 200 μM) manner. GA not only induced DNA damage but also prohibited the DNA repair by altering the DNA repair genes. The telangiectasia mutated, ataxia-telangiectasia and Rad3-related, O6-methylguanine-DNA methyltransferase, DNA-dependent serine/threonine protein kinase, and p53 mRNA expressions were varied in GA treated PC3 cells. This was followed by the investigation of suppression effect of GA on migration and invasion of PC-3 human prostate cancer cells.38 The obtained results specified a dose dependent inhibition of invasion and migration of PC-3 cells. There was blocking of p38, JNK, PKC and phosphatidylinositol 3-kinase (P13K)/AKT signaling pathways of GA treated PC3 cells. This consequently led to the inhibition of MMP-2 and -9 of the PC3 cells.
Agarwal et al. identified GA as one of the phenolic compound with anticancer property. GA had a very strong dose- and time-dependent growth inhibition on DU145 cells. The GA derivatives were also found to cause apoptosis of DU145 cells.39 After this the efficacy and mechanism of GA against DU145 was studied.40 GA (0.3% w/v) caused cell cycle arrest and apoptosis of DU145 cells in time and dose dependent manner. The ATM pathway played a major role in causing cell cycle arrest. Increase in cdc25A/C-cdc2 phosphorylation was eminent in GA treated cells. Next the procyanidin B2-3,3′-di-O-gallate was identified as a major active compound causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells.41 The 3,3′-di-O-gallate ester of procyanidin dimer B2 (Epi–Epi) exhibited dose-dependent effect on DU145 cells. Structural studies the importance of three hydroxyl groups of GA for the antitumor property. Taken together, these data identify procyanidin B2-3,3′-di-O-gallate as a novel biologically active agent against PCA. In continuation, the chemopreventive effects of oral GA feeding on tumor growth and progression in adenocarcinoma of the mouse prostate model TRAMP mice were examined.42 The in vivo experiment showed a decrease in proliferative index with an increase in the apoptotic cells in GA fed TRAMP mice. Doses of GA completely diminished the expression of Cdc2 in the prostatic tissue with strong decrease in the expression of Cdk2, Cdk4, and Cdk6 and the protein levels of cyclin B1 and E.
GA exhibited anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice.43 GA decreased cell viability and induced apoptosis in a dose-dependent manner in both DU145 and 22Rv1 cells. There was also significant inhibition of tumor cell proliferation, induction and reduction of microvessel density in tumor xenografts of 0.3% w/v of GA-fed mice. Suda et al. reported the antitumor activity of procyanidin B2 and B3 gallate derivatives on PC3 cell lines. The derivatives 3-O-gallate, 300-O-gallate, and 3300-di-O-gallate were synthesized and tested for their anticancer property. After the treatment of these derivatives, the cell proliferation was relatively reduced which confirmed the cytotoxity of the GA derivatives.44
5.3 Anticancer activity on hepatic cancer cells
Han et al. experimented the GA induced human hepatoma SMMC-7721 cells apoptosis and its mechanism.45 The in vitro study on SMMC-7721 cells treated with GA (IC50: 50 μM L−1) showed notable inhibition of cell proliferation and induced apoptosis in a dose-dependent manner with nuclear condensation and fragmentation. The mechanism underlying the apoptosis of hepatoma cells was associated with improvement of tumor suppressor gene p53 expression. GA was found to have selective cytotoxicity in rat hepatoma dRLh-84 cells.46 Moreover, they also experimented the effect of GA on normal cells like hepatocytes, macrophages, fibroblasts and endothelial cells after 6 h. Rat hepatoma cells dRLh-84 were entirely killed in 6 h whereas it has no effect in hepatocytes and macrophages. Even, the IC50 for fibroblast and endothelial cells were almost three times as dRLh-84. The IC50 concentration of GA tested is about 4.8–13.2 μg mL−1. The selective cytotoxicity of GA was mainly considered to have structural relationship. The three phenolic hydroxyl group of GA played the major role in inducing cell death by causing cell cycle arrest. The hydroxyl division helped in the implication of differentiating the normal cells from cancer cells. Ohno et al. investigated cytotoxic activity of GA against liver P-815 mastocytoma cells. P815 cells are known to metastasize particularly to the liver. The DBA/2 mice were injected with P815 cells followed by the treatment of GA (IC50: 6.5 μg mL−1). There was a decrease in the number of nodules in the liver and serum glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT), which usually progresses during liver metastasis. GA treatment extended the life span of the DBA/2 mice.475.4 Anticancer activity on melanoma cells
The natural antioxidant GA showed a significant inhibition of cell proliferation and induction of apoptosis in A375S2 human melanoma cells.48 The percentage of viable cells after the treatment of GA decreased in a dose- and time-dependent manner. The molecular mechanism of apoptosis observed, included up-regulation of the proapoptotic Bax proteins but down-regulation of anti-apoptotic Bcl-2 proteins. GA decreased the level of mitochondrial membrane potential in a time-dependent manner and triggered cytosolic release of cytochrome c, promoting the activation of caspase-9 and caspase-3, ultimately leading to apoptotic cell death. In addition, GA promoted release of apoptosis-inducing factor (AIF) and endonuclease G (Endo G). Thus, apoptosis was induced through a caspase-independent pathway. Lo et al. examined the influence of GA on the protein levels and gene expression of matrix metalloproteinases (MMPs) and in vitro migration and invasiveness of human melanoma cells.49 GA treatment decreased the MMPs associated signal pathway protein and MMPs mRNA levels in A375S2 cells. This supported that GA has antimetastatic potential. Moreover, this was involved in the Ras, p-ERK signaling pathways were involved leading to the inhibition of MMP-2 in A375S2 human melanoma cells.The topical application of GA on Swiss albino mice with skin cancer induced by the dimethylbenz[a]anthracene (DMBA)/croton oil was investigated. The volume of the tumor decreased during the experimental period. GA co-treatment with croton oil also exhibited a significant protection by reversal of the altered levels of LDH-isoenzymes, antioxidants, collagen and MMP-2/MMP-9 activities. This study indicates that topical application of GA inhibits skin cancer by modulating the antioxidants and MMPs (2 & 9) in the mouse skin.50 Locatelli et al. reported the antitumoral properties of GA ester derivatives in melanoma cells.51 The octyl, decyl, dodecyl and tetradecyl gallates induced cell death through apoptosis on B16F10 cells. All compounds induced cytotoxic effects, and the IC50 values obtained were between 7 μM and 17 μM after 48 h of incubation. The gallate treatment caused the production of free radicals, depletion of both glutathione (GSH) and ATP, activation of NF-kappaB and inhibition of cell adhesion. The gamma-glutamylcysteine synthase activity played a vital role in GSH depletion. The growth suppression was due to consequence of oxidative stress, resulting in different mechanisms. Other important effects related to the octyl, dodecyl and tetradecyl gallates is related to cell migration and adhesion, by inhibiting the expression of ICAM-1 and VCAM-1 adhesion proteins. Yet another study of anticancer activity of GA derivative lauryl gallate (LG) towards chemically induced skin tumours in IRC mice was done.52 In this study, the application of LG not only selectively destroyed the chemically induced tumours but prevented the formation also. LG inhibited the proto-oncogene tyrosine-protein kinase Src (PTK-c-Src) in the tumor induced by 12-dimethylbenz[a]anthracene (DMBA) and phorbol-12-myristate-13-acetate (PMA) in the mice. The selective toxicity of LG was also confirmed at high doses of 100 μg and 250 μg and for longer period.
5.5 Anticancer activity on colon cancer cells
Yoshioka et al. studied the antitumor effect of GA on human colon adenocarcinoma COLO 205 cells.53 After GA treatment caused the fragmentation of DNA to oligonucleosomal fragments. Morphological changes include appearance of apoptotic bodies showed that GA induced apoptosis. GA had a concentration dependent and time dependent effect on the COLO 205 cells. GA was found to exhibit anticancer property against HCT-15 human colon cancer cells.54 It reduced the cell viability of the colon cancer cells in dose dependent manner. Cell shrinkage, rounding of cells and detachment from the substratum were the prominent changes in GA treated cells. The IC50 concentration of GA experimented is 96 μg mL−1. Khaled et al. reported the antioxidant and cytotoxic effect of GA-based indole derivatives at concentration of 19.2 μM. The cytotoxic activity of the compounds was evaluated against HCT-116 human colon cancer cell line. There was decrease in the cell viability after the GA derivatives treatment. It was found that antioxidant property and structure of GA derivates displayed a similar relationship.555.6 Anticancer activity on lymphoma cells
GA was found to inhibit the cell viability of the human monocytic lymphoma cell line U937.56 The in vitro experiments showed that GA induced apoptosis in the lymphoma cell line in a dose dependent manner. After the treatment of GA, upregulation of NF-κB protein and down-regulation of the proliferating cell nuclear antigen and IkappaB kinase (I-κB) protein. These results demonstrate that GA as a potential chemotherapeutic agent for lymphoma. Serrano et al. studied the effect of GA and its alkyl esters (methyl, propyl, octyl, and lauryl at IC50: 40, 35, 12, 1.5, 1 μM respectively) on mouse B cell lymphoma Wehi 231 cell line and blood lymphocytes.57 The Wehi 231 cell lysis was observed after the treatment of GA and its ester derivatives. There was DNA fragmentation in the lymphoma, which is one of the classical biochemical changes during apoptosis. There were also other morphological changes like cell shrinkage, chromatin condensation and presence of apoptotic bodies leading to cell death. On the other hand, the blood lymphocytes were viable after the treatment of GA and its derivatives.This was continued with the study on mechanistic aspects of the induction of apoptosis by lauryl gallate, one of the alkyl ester of GA in the murine B-cell lymphoma line Wehi 231.58 This compound inhibited the protein tyrosine kinases (PTKs) in whole cells. Long-term treatment showed the changes in the functions of mitochondria in relation with release of cytochrome c and increase of mitochondrial transmembrane potential. This led to activation of caspases with breakdown of DNA. The study also revealed that the proapoptotic effect of lauryl gallate is not dependent on over expression of Bcl-2.
5.7 Anticancer activity on leukemia
Madlener et al. recently studied the cytotoxic and biochemical effects of GA on human HL 60 promyelocytic leukemia cell line.59 Apoptosis of leukemia cells was evident after the treatment of GA (IC50: 80 μM), which was accompanied with the cell cycle arrest at the G0/G1 phase. GA also caused the inhibition of ribonucleotide reductase. Similarly, Yeh et al. reported that GA had an antiproliferative activity on HL 60 cells.60 The GA caused the DNA damage and fragmentation on cancer cells, time- and dose dependently. The apoptosis induced was in relation with mitochondrial pathway by promoting the release of cytochrome c, apoptosis-inducing factor (AIF) and endonuclease G (Endo G), up-regulation Bcl-2 protein activating caspase-4, caspase-9 and caspase-3. In addition, the death receptors also participated in GA induced apoptosis.The role of ROS generation in apoptosis induced by GA on promyelocytic leukemia HL-60RG cells was reported.61 The generation of ROS in the GA treated HL 60 cells was dose dependent. The intracellular peroxide level was well correlated with the potency to induce apoptosis after the GA treatment. The role of ROS generation causing apoptosis was prominent than the activity of intracellular peroxide level. GA had an anti-leukemic effect on the human leukemia K562 cells.62 The cell viability of GA treated K562 cells were in dose- and time-dependent manner. GA with concentration of 4 μM (IC50) caused the G0/G1 phase arrest by inhibiting the cyclin D and cyclin E levels. There was release of cytochrome c and PRAP cleavage along with DNA fragmentation. The caspase-3 was up regulated in the cells. GA also inhibited BCR/ABL tyrosine kinase and NF-κB. Thus, the cell death due to GA treatment involves death receptor and mitochondrial-mediated pathways by inhibiting BCR/ABL kinase, NF-κB activity and COX-2.
The ester derivatives of GA had the ability to induce apoptosis through the DNA ladder fragmentation pattern on murine lymphoblastic L1210 leukemia cells.63 There was also mitochondrial and cytoplasmic GSH depletion and NF-kappaB activation. This study also reflected relationship between cytotoxic effect and a limited degree of lipophilicity. The octyl- and lauryl gallates had high potent to induce apoptosis on HL 60 cells compared to ethyl-, propyl- and butylgallates.64 Octylgallate markedly inactivated aconitase and generated ROS leading to apoptotic cell death. The gallate treatment caused generation of reactive oxygen species through the redox cycling in cells, resulting in the induction of apoptosis. The other GA derivatives such as 3,4-methylenedioxyphenyl 3,4,5-trihydroxybenzoate (GD-1) and S-(3,4-methylenedioxyphenyl) 3,4,5-trihydroxythiobenzoate (GD-3) with concentration of 14.5 and 3.9 μM respectively; also induced cell death of promyelocytic leukemia HL-60RG cells.65 After treatment poly (ADP-ribose) polymerase (PARP), a substrate of caspase-3, was cleaved with increasing incubation time. The GA derivatives activated caspase-3 following intracellular Ca2+ elevation independent of reactive oxygen species cumulatively leading to cell death.
The (−)-epigallocatechin-3-gallate (EGCG), was tested for their antitumor property with K562 leukemia cells. It was found to be DNA topoisomerase poison and it may be categorized as anticancer drug. High levels of topo I- and topo II-DNA complexes were observed in K562 leukaemia cells exposed to EGCG. These changes caused by EGCG were in a time dependent manner and selectively killed tumor cells.66 The ester derivative lauryl gallate inhibited the cell proliferation of HL60 and KG-1 cells. The gallate had a time and dose dependent effect on the cancer cells. It induced the activation of both extrinsic and intrinsic apoptotic pathways, involving dissipation of mitochondrial membrane potential, down regulation of anti-apoptotic proteins (Bcl-2, Mcl-1, and Bcl-xL), up regulation of pro-apoptotic proteins (Bak, PUMA, DR4, and DR5), and increased caspase-2, -3, -8, and -9 activation.67 In contrast, the other derivative propyl gallate (PG) induced apoptosis, which involved the regulation of ROS signaling. PG (IC50: 75 μM) reduced cell viability in HL-60 leukemia cells by activating caspases-3, -8, and -9 and increased the levels of p53, Bax, and Fas ligand. There was also an early event of PG-induced apoptosis, which is MAPKs/Nrf-2-mediated GSH depletion in the gallate treated cells.68
5.8 Anticancer activity on esophageal cancer cells
Faried et al. studied the anticancer activity of GA on esophageal cancer cells TE-2.69 GA demonstrated a significant antiproliferation in TE-2 cells except CHEK-1 cells (noncancerous). The molecular mechanism observed in GA induced apoptosis was up-regulation of the pro-apoptosis Bax protein activity in cancer cells. On the other hand, GA down-regulated anti-apoptosis proteins such as Bcl-2 and Xiap along with the survival Akt/mTOR pathway. In contrast, the expression of pro-apoptosis related proteins was delayed in non-cancerous cells. There were also noticeable morphological changes in the TE-2 cells after 12 h of treatment, which was missing in the normal CHEK-1 cells. The effects of the combination of GA derivatives epigallocatechin-3-gallate (EGCG) or theaflavin-3-3′-digallate (TF3) with ascorbic acid (Vc) on esophageal carcinoma Eca-109 cells was reported.70 The results showed that Vc could enhance the EGCG and TF3 induced apoptosis in Eca-109 cells. This effect concerned the activation of caspase-3 and -9. EGCG, TF3 and Vc could activated MAPK pathways and each compound activated diverse MAPK subfamilies in the cells. The mechanism of action of (−)-epigallocatechin-3-gallate (EGCG) on growth inhibition in human esophageal squamous cell carcinoma KYSE 150 cells was noted by Hou et al.71 The findings suggest that in cell culture conditions, the autoxidation of EGCG (IC50: 20 μM L−1) leads to epidermal growth factor receptor (EGFR) inactivation, but the inhibition of cell growth is due to other mechanisms. EGCG treatment caused decrease of HER-2/neu signals. It remains to be determined whether the presently observed autoxidation of EGCG also occur in vivo conditions.5.9 Anticancer activity on cervical cancer cells
You et al. evaluated the effects of GA on HeLa cervical cancer cells and human umbilical vein endothelial cells (HUVEC) in relation to cell growth inhibition and death. GA was found to induce cell lysis in both the cell type. This cell death was accompanied by the loss of mitochondrial membrane potential in the cervical cancer cells. There was also increase in the ROS generation and GSH depletion in the HeLa cells treated with GA. HeLa cell growth was diminished with an IC50 of approximately 80 μM GA at 24 h whereas an IC50 of GA in HUVEC cells was approximately 400 μM showing the selectivity.72 This was closely followed by the study on the effects of mitogen-activated protein kinase (MAPK) inhibitors or small interfering RNAs (siRNA) on GA of same concentration induced HeLa cell death in relation to reactive oxygen species (ROS) and glutathione (GSH).73 GA inhibited the growth of HeLa cells in a dose dependent fashion with the loss of mitochondrial membrane potential, increase the ROS level including O(2)(˙−) and significant GSH depletion. GA reduced the activity of ERK and increased the activity of JNK at the same time. Additionally, p38 siRNA administration augmented growth inhibitions in GA-treated HeLa cells. In another independent study by Zhao et al., GA was reported to reduce the cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Treatment of HeLa human cancer cells with GA (IC50: 10 μg mL−1) decreased cell viability in a dose-dependent manner. It was also observed that GA decreased the HeLa cell proliferation. In comparison with the cytotoxic effect on the HeLa and HTB-35 cervical cancer cells, GA exhibited less cytotoxicity in normal HUVECs. GA reduced cell viability to ∼92, 84 and 66% of the control in the HeLa cells and to ∼94, 88 and 64% of the control in the HTB-35 cells at concentrations of 5, 10 and 15 μg mL−1, respectively. However, at the same concentrations, GA decreased the cell viability to ∼120, 111 and 75% of the control, respectively, in the HUVEC cells. The prevention of cell invasion was considered due to suppression of ADAM17 and the down regulation of the EGFR, Akt/p-Akt and Erk/p-Erk signaling pathways. Angiogenesis is the formation of new blood vessels, which is considered a critical step for the growth of solid tumors. Due to the neovascular nature of cervical cancer, the ability of GA in relation with angiogenesis was studied. To investigate this, the effects of GA to inhibit the tube formation in HUVECs were performed. The results showed significant inhibition of the elongation of the tubes at all concentrations, and also the tube length per area was decreased by GA treatment.74The GA derivatives (−)epigallocateocatechin-3-gallate (EGCG) and theaflavins (TF) was tested for its anticancer property against human cervical cancer cells HeLa and SiHa.75 Cells were treated with EGCG or TF (IC50: 25 & 30 μg mL−1 respectively) and cisplatin (CDDP) alone and with their combinations. The combined treatment of EGCG or TF with CDDP elicited cell death by apoptosis in both the cell types. The apoptosis involved the inhibition of Akt and NF-κB through blocking phosphorylation of inhibitor kappa Bα with increase in ROS level, release of cytochrome-c and decrease in cellular glutathione contents and Bcl-2 expression, eventually resulting in the activation of caspases, poly(ADP)ribose polymerase cleavage and apoptosis of cancer cells. The GA derivative propyl gallate (PG) was found to inhibit the growth of HeLa cervical cancer cells.76 The cell growth inhibition and apoptosis of PG treated cells was in a dose dependent manner. There was a change in the intracellular ROS levels including O(2)(−) were observed in PG-treated HeLa cells depending on the incubation time and doses. There was also involvement of glutathione (GSH) which was dominant than the changes of ROS level. In addition, PG induced cell cycle arrest in G1 phase of HeLa cells.
5.10 Anticancer activity on oral carcinoma cells
GA, the plant polyphenol was said to have cytotoxic and proapoptotic activities on human oral cancer HSC-2 cells.77 Human oral carcinoma HSC-2 cells were more sensitive to GA than normal human gingival fibroblasts. The cytotoxicity was observed at 80 μM for the HSC-2 cells and 175 μM for HF-1 fibroblasts with 24 h exposure. The GA decreased intercellular glutathione, caused lipid peroxidation and increased the level of intracellular reactive oxygen species. The apoptosis in the HSC-2 cells was in concentration-dependent manner. Overall, the cytotoxicity was due to induction of oxidative stress leading to apoptosis of cells. This Chia et al. examined the anti-neoplastic effects of GA on oral squamous carcinoma HOSCC cells.78 The viability of the HOSCC cells was reduced after the treatment. There was up regulation of pro-apoptotic genes like TNF-α, TP53BP2, and GADD45A along with the down regulation of the anti-apoptotic gene Survivin and cIAP1. This showed that GA induced apoptosis cell death in HOSCC cells.The information regarding the effect of GA on cell migration and invasion of human oral squamous carcinoma SCC-4 cells was reported by Kuo et al.79 GA reduced the migration and invasion of SCC4 cells by reducing the translocation of NF-κB and RhoA from the cytosol to the nucleus. There was also inhibition of matrix metalloproteinase (MMP)-2 and MMP-9 activity. This supported GA as a therapeutic agent for oral cancer.
5.11 Anticancer activity on other cancer cells
Liang et al. conducted both in vivo and in vitro studies on the antiproliferative effect of GA against U-2OS osteosarcoma cells.80 GA inhibits the proliferation of human osteosarcoma cells in a time- and dose-dependent manner through apoptosis. The levels of p-JNK and p-ERK1/2 kinase decreased while the level of p-p38 kinase increased after the treatment with GA. This indicated that GA induced apoptosis of osteosarcoma cells through the inactivation of JNK and ERK1/2 kinase pathways and the activation of p38 kinase pathway. In the in vivo condition, GA treatment inhibited MNNG/HOS tumor xenograft growth in a time-dependent fashion. The GA prohibited the tumor growth by decreasing the proliferation, inhibiting angiogenesis, and promoting apoptosis. The mechanisms of GA in opposition to migration and invasion of human osteosarcoma U-2 OS cells recently reported. The CD31, a tumor angiogenesis marker was significantly less showing the anti-angiogenesis effect of GA in U-20S cells.81 GA decreased the protein of GRB2, PI3K, AKT/PKB, PKC, p38, ERK1/2, JNK, NF-κB p65 and inhibited the activities of AKT, IKK and PKC in the osteosarcoma cells. In addition, there was decrease in MMP-2 and MMp-9 proteins leading to mitogen-activated protein kinase (MPAK).GA, the natural polyphenolic acid, possessed antitumor effects of GA on MCF-7 breast cancer cell.82 The GA treatment lessened the cell growth of MCF-7 cells in a dose-dependently (IC50: 10 μg mL−1). The levels of cyclin A, CDK2, cyclin B1 and cdc2/CDK1 were diminished while the levels of the negative regulators p27(Kip1) and p21(Cip1) were increased by GA treatment. These resulted to the accumulation of cells in G2/M phase arrest in MCF7 cells. The ester derivative of GA, lauryl gallate was tested for its antiproliferative effect on estrogen-dependent MCF7 cells and estrogen independent MDA-MB-231 and MCF7 ADR cells.83 The lauryl gallate (IC50: 0.5–10 μM) altered the proliferation and cell cycle of all the three types of breast cancer cells. Cell cycle arrest in the G1 phase of gallate treated MCF 7 cells along with increase of p53 expression. There was a slowdown of cell proliferation and up regulation of p21Cip1 and reduced cyclin D1 levels in all three-breast cancer cell lines. The induction of apoptosis involved PARP cleavage and mitochondrial membrane depolarization and morphological alteration after lauryl gallate treatment. Over expression of Bcl-2 in MCF7 ADR cells was also observed.
Kang et al. studied the effect of GA on PC12 rat pheochromocytoma cell.84 GA reduced the cell viability dose dependently (IC50: 50 μM L−1). It caused the cleavage of poly (ADP-ribose) polymerase. The GA treatment also caused phosphorylation of c-Jun N-terminal protein kinase (JNK) and the down regulation of Bcl-2 in PC12 cells. Thus, GA induced apoptosis in the PC12 cells. GA exhibited anti-metastasis effect on gastric adenocarcinoma (AGS) cell metastasis.85 GA induced some level of cell toxicity with inhibition of MMP 2/9 expression. Multiple proteins involved in metastasis and the cytoskeletal reorganization signal pathway, including Ras, Cdc42, Rac1, RhoA, RhoB, PI3K and p38MAPK, were also inhibited by GA (IC50: 0.01 mM).
Ou et al. tested the effect of GA on human bladder transitional carcinoma cells. GA (IC50: 40 μM) regulated the cell cycle of the carcinoma cells.86 There was significant increase in G2/M phase cells, accompanied by decrease in G0/G1 phase cells after GA treatment. GA caused the decrease of cyclin-dependent kinases (Cdk1), Cyclin B1 and Cdc25C, but increase of p-cdc2 (Tyr-15) and Cip1/p21 and phosphoeylation of Cdc25C at Ser-216 in dose dependent manner. This consequently leads to its translocation from nucleus to cytoplasm. GA exhibited selective antiproliferative effect of GA on human pancreatic cancer cell lines CFPAC-1 and MiaPaCa-2 in comparison to the normal hepatocytes HL-7702 cells. GA inhibited the proliferation of CFPAC-1 and MiaPaCa-2 cells in a time- and dose-dependent manner, with IC50 of 102.3 ± 2.4 and 135.2 ± 0.6 μM. GA treatment activated caspase-3, caspase-9, and ROS, elevated Bax expression and reduced mitochondrial membrane potential of the cancer cells compared to the hepatocytes.87 The rat multi-organ carcinogenesis model was treated with propyl gallate, another GA derivative and the observations were reported. Intra-gastric administration of propyl gallate was effective in reducing the multiplicity of kidney atypical tubules.88
6. Conclusion
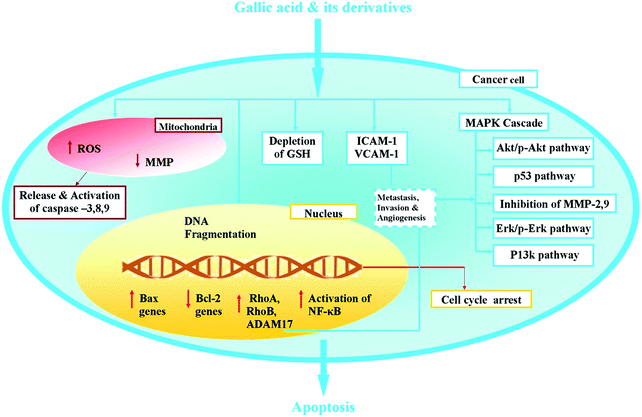
Cancer is a generic term for a large group of diseases that can affect any part of the body. It is one of the leading causes of death worldwide.1 A significant proportion of cancers can be cured by surgery, chemotherapy or radiotherapy. Chemotherapy is a category of cancer treatment that uses chemical substances, especially anti-cancer drugs.6 It is employed before and after surgery and in combination with radiotherapy. This technique has a range of side effects and also it cannot cure cancer when detected at latter stages which leads to continuous development of anticancer drugs. More importance is being given to natural compounds with anticancer property. In this review, GA and its derivatives are proposed as one of the prominent candidates for treating cancer.The GA and its derivatives are found to be active against lung cancer, colon cancer, breast cancer, prostate cancer, esophageal cancer, hepatoma, lymphoma, leukemia, osteosarcoma and melanoma cells. They induce programmed cell death in the malignant cells either dose dependently or time dependently. The notable changes seen after the treatment are generation of ROS, regulation of apoptotic and anti-apoptotic proteins, suppression and promotion of oncogenes, inhibition of matrix metalloproteinase (MMPs), activation of caspase-3, caspase-8, caspase-9, p53, and c-Jun N-terminal kinases (JNK) signaling pathways and cell cycle arrest in the G0/G1/M phase of cell cycle. GA and its derivatives are found to inhibit invasion and metastasis. Metastasis is the spread of a cancer from one organ or part to another part or organ that is not directly connected to it, while invasion of cancer specifies the spread or advent of cancer from its point of origin into surrounding tissues. Invasion and metastasis can be facilitated by proteins, which stimulate tumor cell attachment to host cellular or extracellular matrix determinants and tumor cell proteolysis of host barriers. The matrix metalloproteinases (MMPs) plays an important role of cell invasion capable of degrading a range of extracellular matrix proteins allowing cancer cells to migrate and invade.89 It was reported that inhibition of MMP-2 and MMP-9 are involved with the non-metastatic potential of GA in hepatic and gastric cancer cell lines46,84 along with anti-invasion of GA in prostrate, cervical and oral cancer cell lines.37,73,74,78 GA also down regulates ICAM-1 and VCAM-1 adhesion proteins responsible for migration and invasion mechanisms that occur in the metastatic tumor of the melanoma cells.51 These were accompanied with regulation of the cytoskeletal reorganization pathways including Ras, p38MPAK and P13K along with the signaling pathways such as Akt and Erk as well as modulation of metallopeptidase genes RhoA, RhoB and ADAM17 GA. The major observations of effects caused by GA and its derivatives in each cancer type are summarized in Table 1 and diagrammatically represented in Fig. 3.
| Type of cancer | Cell lines tested | Major alterations after treatment | References |
|---|---|---|---|
| Lung | SBC-3 small cell carcinoma | • Dose dependent effect | 30–34 |
| EBC-1 squamous cell carcinoma | • DNA fragmentation | ||
| A549 adenocarcinoma cells | • ROS generation | ||
| LL-2 murine Lewis lung carcinoma cells | • Deceases of glutathione | ||
| Calu-6 lung carcinoma cell cells | • Activation of c-Jun NH2-terminal kinase (JNK) | ||
| • Activation of caspase-3 | |||
| Prostrate | DU145 human prostate cancer cells | • Induction of mitochondrial mediated apoptosis | 35–43 |
| PC3 human prostate cancer cells | • Generation of ROS | ||
| 22Rv1 human prostate carcinoma epithelial cells | • Decrease of cyclin B1 and E | ||
| • Cell cycle arrest at G2/M phase | |||
| • Release of cytochrome c | |||
| • Alteration of DNA repair gene | |||
| • Blockage of phosphatidylinositol 3-kinase (P13K)/AKT signaling pathways | |||
| Hepatic | SMMC-7721 human hepatoma cells | • Inhibition of cell proliferation | 45–47 |
| dRLh-84 rat hepatoma cells | • Decrease in liver and serum glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) | ||
| P-815 liver mastocytoma cells | • Induction of apoptosis dose dependently | ||
| Skin | A375S2 human melanoma cells | • Up-regulation of Bax proteins | 48–52 |
| • Down-regulation of Bcl-2 proteins | |||
| • Release of apoptosis-inducing factor (AIF) & endonuclease G (Endo G) | |||
| • Decrease of matrix metalloproteinases (MMPs) related proteins | |||
| • Depletion of GSH | |||
| • Inhibition of proto-oncogene tyrosine-protein kinase Src (PTK-c-Src) | |||
| • Decrease of cell viability | |||
| Colon | COLO 205 human colorectal carcinoma cells | • Cell shrinkage | 53–55 |
| HCT 15 human colon adenocarcinoma cells | • Appearance of apoptotic bodies | ||
| HCT 116 human colon cancer cells | • Oligonucleosomal DNA fragments | ||
| • Concentration and time dependent cell death | |||
| Lymphoma | U937 human monocytic lymphoma cells | • Up-regulated the NF-κB protein | 56–58 |
| Wehi 231 murine B-cell lymphoma cells | • Down-regulated the proliferating cell nuclear antigen and IkappaB kinase (I-κB) protein | ||
| • Chromatin condensation | |||
| • DNA fragmentation | |||
| • increase of mitochondrial transmembrane potential | |||
| Leukemia | HL 60 human promyelocytic leukemia cells | • Cell cycle arrest at the G0/G1 phase | 59–68 |
| HL-60RG promyelocytic leukemia cells | • Inhibition of ribonucleotide reductase | ||
| K562 human leukemia cells | • DNA damage and fragmentation time- and dose dependently | ||
| L1210 murine lymphoblastic leukemia cells | • Release of cytochrome c, apoptosis-inducing factor (AIF) & Endo G | ||
| KG-1 human acute myeloid leukemia cells | • Up-regulation Bcl-2 protein | ||
| • Activation of caspase-4, -9 & -3 | |||
| • Prominent activity of intracellular peroxide levels | |||
| • PRAP cleavage | |||
| • Inhibition of BCR/ABL tyrosine kinase | |||
| • Activation of NF-κB | |||
| • GSH depletion | |||
| • Increased levels of p53, Bax & Fas ligand | |||
| Esophageal | TE-2 esophageal cancer cells | • Up-regulation of Bax protein activity reduction of Bcl-2 and Xiap proteins activity | 69–71 |
| • Down regulation of survival Akt/mTOR pathway | |||
| • Activation of caspase-3, -9 and MAPK pathway | |||
| • Inactivation of epidermal growth factor receptor (EGFR) | |||
| Cervical | HeLa cervical cancer cells | • Decrease in mitochondrial membrane potential | 72–76 |
| • Depletion of GSH | |||
| • Down regulation of the EGFR, Akt/p-Akt and Erk/p-Erk signaling pathways | |||
| • Activation of caspases & poly(ADP)ribose polymerase cleavage | |||
| • Cell cycle arrest in G1 phase | |||
| Oral | HSC-2 human oral carcinoma cells | • Reduction of GSH | 77–79 |
| HOSCC oral squamous carcinoma cells | • Lipid peroxidation | ||
| SCC-4 human oral squamous carcinoma cells | • Increased the level of intracellular reactive oxygen species | ||
| • Up regulation of TNF-α, TP53BP2, and GADD45A | |||
| • Inhibition of matrix metalloproteinase (MMP)-2 and MMP-9 | |||
| Bone | U-2OS oesteosarcoma cells | • Decrease in levels of p-JNK and p-ERK1/2 kinase | 80 and 81 |
| • Increase in level of p-p38 kinase | |||
| • Time- and dose- dependent apoptosis | |||
| • Decrease in MMP-2 and MMp-9 proteins | |||
| Breast | MCF-7 breast cancer cells | • Increase of p27(Kip1) and p21(Cip1) negative regulators | 82 and 83 |
| MDA-MB 231 human breast adenocarcinoma cells | • Increase of p53 expression | ||
| • Slowdown of cell proliferation dose dependently | |||
| • Up regulation of p21Cip1 | |||
| • Reduction in cyclin D1 | |||
| • Induction of PARP cleavage | |||
| Neuro-endocrine | PC12 rat pheochromocytoma cells | • Cleavage of poly (ADP-ribose) polymerase | 84 |
| • Phosphorylation of c-Jun N-terminal protein kinase (JNK) | |||
| • Down regulation of Bcl-2 | |||
| Gastric | AGS gastric adenocarcinoma cells | • Inhibition of proteins involved in MMP 2/9, metastasis & cytoskeletal recognition | 85 |
| Bladder | TCC human bladder transitional carcinoma cells | • Increase in G2/M phase cells | 86 |
| • Decrease in G0/G1 phase cells decrease of cyclin-dependent kinases (Cdk1) | |||
| • Phosphoeylation of Cdc25C | |||
| Pancreas | CFPAC-1 human caucasian pancreatic adenocarcinoma cells | • Activation of caspase-3, -9 | 87 |
| MiaPaCa-2 human pancreatic carcinoma cells | • ROS generation | ||
| • Increase in Bax expression |
GA is found to have anti-angiogenesis property in cervical cancer and osteosarcoma cells.74,81 Their results did not depict much about the mechanism involved. In comparison, The GA present in the extract of Rubus leaf extract and Toona sinensis leaf extracts were reported to inhibit vascular endothelial growth factor (VEGF), related to angiogenesis.90,91 Hence, more studies to understand the mechanism of anti-angiogenesis property of GA should be performed. NF-κB is a protein that is important in providing immune response to various viral infection and inflammation. NF-κB moves from the cytoplasm into the nucleus and promotes cancer cell proliferation, angiogenesis, and metastasis.92 It is fascinating to see how the GA interacts with NF-κB. From this review, it has been inferred that GA could both inhibit and activate NF-kB. To summarize, GA inhibited the NF-κB of lymphoma (K562), osteosarcoma and cervical cancer cells62,75,81 and induced apoptosis by through mitochondrial mediated pathway involving the modulation of Bcl2/Bax ratio and activation of caspase-3. Contrastingly, in some studies GA found to activate the NF-kB in the lymphoma (U937) and oral carcinoma cells.56,79 In this case, this is followed by the activation of caspase-8 and Fas mediated apoptosis. This paradoxical behavior of GA may be attributed to the type of cancer cell investigated. However, more experiments to delineate this effect of GA should be performed to explain the role of NF-κB in GA-mediated cell death.
It also has been reported that the cell death promoted by GA and its derivatives, in different cell lines may be related with glutathione (GSH) depletion. Since the intracellular GSH has a decisive effect on anticancer drug-induced apoptosis, the reduction of GSH levels by GA and its derivatives may related with the drug resistance-reversal activity. If used in combination with anti-cancer drugs that are already in usage, it would enhance the success of the treatment. Multidrug-resistance (MDR) is the chief limitation to the success of chemotherapy. According to the National Cancer Institute, multidrug-resistance is a phenomenon where cancer cells adopt to anticancer drugs in such a way that drugs become less effective. Cancer cells adopt several mechanisms to evade death induced by anticancer agents. These cells develop resistance by increased expression of multidrug-resistant proteins, which alters anti-cancer drug transport mechanisms. Among these proteins, P-glycoprotein (Pgp, ABCB1) and multidrug resistance-associated protein (MRP1, ABCC1) are the prime ones. Thus, for proper understanding of GA's activity in drug resistance-reversal, more studies on interaction of GA and its derivatives with these Pgp proteins, should be performed.
Apart from this, the depletion of GSH and generation of ROS by GA may also be related to other form of cell death, which needs further investigation. To list, depletion of GSH combined with the deactivation of glutathione peroxidase 4 (GPx4) and the resulting oxidative stress have been linked to ferroptosis.93 Ferroptosis is a newly emerged form of iron dependent cell death that is totally different from apoptosis, necrosis and autophagy, morphologically, biochemically and genetically. This type of cell death is characterized by the iron-dependent accumulation of lethal lipid ROS. ROS accumulation claims to be an essential factor in all forms of apoptotic and non-apoptotic death. Ability of GA to induce ferroptosis can be elucidated by its interaction with ferroptosis inhibiting compound like ferrostatin-1,94 which may shed more light about this putative role. Similarly, it will be interesting to explore whether GA has any role in autophagy. Autophagy is an intracellular degradation system that delivers cytoplasmic constituents to the lysosome. The role of autophagy in cancer cells is extensively researched, which emphasizes autophagy as tumor suppressor as well as a factor for tumor survival.95 Hence, the relationship between GA and autophagy should also be inspected to have better understanding about GA-induced cell deaths.
Even though, there has been several in vitro as well as quiet few in vivo experiments on the anticancer property of GA and its derivative in cancer cells, more efficient information would be obtained with the knockout mouse (genetically engineered mouse). It is high time that human clinical trials on healthy subjects and subjects with pre-existing medical conditions should be done. More studies regarding the absorption of GA during oral administration in the form of tablets or free form should be done. In addition to this, investigation on other different modes of administration may also be carried out. The observations should be made, once the subjects are treated with GA and its derivative at various phases. The first phase may start with 10 to 20 individuals and the final phase may involve 1000 to 3000 individuals. This may lead to the more insights resulting in the development of GA and its derivatives as prominent anticancer drugs.
Acknowledgements
This work was supported partly by the Ministry of Higher Education Malaysia with the Grant Vot no. R.J130000.7809.4F444 and also acknowledges the support of UPMU, UTM.Notes and references
- S. Bernard and P. Christopher, World Cancer Report 2014, IARC Nonserial Publication, WHO press, 2014 Search PubMed.
- Cancer Facts & Figures, American cancer society, 2014, http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014, accessed October 2014 Search PubMed.
- S. Rebecca, M. Jiemin, Z. Zhaohui and J. Ahmedin, Ca-Cancer J. Clin., 2014, 64, 9–29 CrossRef PubMed.
- R. Rampling, A. James and V. Papanastassiou, J. Neurol., Neurosurg. Psychiatry, 2004, 75, 24–30 CrossRef.
- M. J. Lind, Medicine, 2008, 36(1), 19–23 CrossRef PubMed.
- H. Joensuu, Lancet Oncol., 2008, 9(3), 304 CrossRef.
- F. A. Ayaz, A. S. Hayirlioglu, J. Gruz, O. Novak and M. Strnad, J. Agric. Food Chem., 2005, 53(21), 8116–8122 CrossRef CAS PubMed.
- H. Wang, G. J. Provan and K. Helliwell, J. Pharm. Biomed. Anal., 2003, 33(4), 539–544 CrossRef CAS.
- J. J. Lua, Y. Weib and Q. P. Yuan, Sep. Purif. Technol., 2007, 55, 40–43 CrossRef PubMed.
- V. U. Borde, P. P. Pangrikar and S. U. Tekale, Recent Res. Sci. Technol., 2011, 3, 51–54 CAS.
- G. Mukherjee and R. Banerjee, Chem. Today, 2003, 21, 59–62 CAS.
- C. Locatelli, F. B. Filippin-Monteiro and T. B. Creczynski-Pasa, Eur. J. Med. Chem., 2013, 60, 233–239 CrossRef CAS PubMed.
- P. E. Thompson, A. M. Moore and J. W. Reinertson, Antimicrob. Agents Chemother., 1953, 3, 399–408 CAS.
- A. Mahadevan and M. K. Reddy, Neth. J. Plant Pathol., 1968, 74, 87–90 CrossRef CAS.
- A. F. Florov and E. L. Mishenkova, Mikrobiol. Zh., 1970, 32, 628–633 CAS.
- L. Claudriana, B. Fabíola, M. Filippin and C. Ariana, Antioxidant, antitumoral and anti-inflammatory activities of Gallic acid, Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications, Chapter: Antioxidant, Antitumoral and Anti-Inflammatory Activities of Gallic Acid, 4th edn, 2013, pp. 1–23 Search PubMed.
- D. M. Nguyen, D. J. Seo, H. B. Lee, I. S. Kim, K. Y. Kim, R. D. Park and W. J. Jung, Microb. Pathog., 2013, 56, 8–15 CrossRef CAS PubMed.
- C. A. van der Heijden, P. Janssen and J. J. Strik, Food Chem. Toxicol., 1986, 24, 1067–1070 CrossRef CAS.
- S. M. Fiuza, C. Gomes, L. J. Teixeira, M. T. Girão da Cruz, M. N. D. S. Cordeiro, N. Milhazes, F. Borges, M. P. M. Marques, K. C. Choi, Y. H. Lee, M. G. Jung, S. H. Kwon, J. Kim, W. J. Jun and J. Lee, Phenolic acid derivatives with potential anticancer properties—a structure–activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids, Mol. Cancer Res., 2009, 7, 2011–2021 CrossRef PubMed.
- K. C. Choi, Y. H. Lee, M. G. Jung, S. H. Kwon, J. Kim, W. J. Jun and J. Lee, Mol. Cancer Res., 2009, 7, 2011–2021 CrossRef CAS PubMed.
- A. C. kaliora, P. T. kanellos and N. kalogeropouls, Chapter: Gallic acid bioavailability in humans, in Handbook on gallic acid, Nova publishers, 2013 Search PubMed.
- F. D. Robbins, O. H. Emerson, F. T. Jones, A. N. Booth and M. S. Masri, J. Biol. Chem., 1959, 234, 3014–3016 Search PubMed.
- Y. Konishi, Y. Hitomi and E. Yoshika, J. Agric. Food Chem., 2004, 52, 2527–2532 CrossRef CAS PubMed.
- S. Shahrzad and I. Bitsch, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 705, 87–95 CrossRef CAS.
- S. Shahrzad, K. Ayogi, A. Winter, A. Koyama and I. Bitsch, J. Nutr., 2001, 131(4), 1207–1210 CAS.
- A. R. Stoddard, L. R. Koetje, A. K. Mitchell, M. P. Schotanus and J. L. Ubels, J. Ocul. Pharmacol. Ther., 2013, 29(7), 681–687 CrossRef CAS PubMed.
- K. Rajalakshmi, H. Devaraj and S. Niranjali Devaraj, Food Chem. Toxicol., 2001, 39, 919–922 CrossRef CAS.
- N. Niho, M. Shibutani, T. Tamura, K. Toyoda, C. Uneyama, N. Takahashi and M. Hirose, Food Chem. Toxicol., 2001, 39, 1063–1070 CrossRef CAS.
- C. A. van der Heijden, P. J. Janssen and J. J. Strik, Food Chem. Toxicol., 1986, 24(10–11), 1067–1070 CrossRef CAS.
- Y. Ohno, K. Fukuda, G. Takemura, M. Toyota, M. Watanabe, N. Yasuda, Q. Xinbin, R. Maruyama, S. Akao, K. Gotou, T. Fujiwara and H. Fujiwara, Anticancer Drugs, 1999, 10(9), 845–851 CrossRef CAS PubMed.
- Y. Ohno, T. Ri, H. Ikoma, T. Yuugetu, M. Asai, N. Watanabe, S. Yasuda, G. Akao, S. Takemura, K. Minatoguchi, H. Gotoh, H. Fujiwara, K. Fukuda and M. Kawada, Anticancer Drugs, 2001, 12(10), 847–852 CrossRef PubMed.
- R. B. You and H. P. Woo, Toxicol. in Vitro, 2010, 24, 1356–1362 CrossRef PubMed.
- Y. C. Chiu, C. C. Kun, Y. Y. Tsung, C. L. Hsiang and L. H. Shih, J. Evidence-Based Complementary Altern. Med., 2013, 1–12 CrossRef PubMed.
- D. K. Maurya, N. Nandakumar and T. P. Devasagayam, J. Clin. Biochem. Nutr., 2011, 48(1), 85–90 CrossRef CAS PubMed.
- H. M. Chen, Y. C. Wu, Y. C. Chia, F. R. Chang, H. K. Hsu, Y. C. Hsieh, C. C. Chen and S. S. Yuan, Cancer Lett., 2009, 286(2), 161–171 CrossRef CAS PubMed.
- L. H. Russell, E. Mazzio, R. B. Badisa, Z. P. Zhu, M. Agharahimi, E. T. Oriaku and C. B. Goodman, Anticancer Res., 2012, 32(5), 1595–1602 CAS.
- K. C. Liu, H. C. Ho, A. C. Huang, B. C. Ji, H. Y. Lin, F. S. Chueh, J. S. Yang, C. C. Lu, J. H. Chiang, M. Meng and J. G. Chung, Environ. Toxicol., 2013, 28(10), 579–587 CrossRef CAS PubMed.
- K. C. Liu, A. C. Huang, P. P. Wu, H. Y. Lin, F. S. Chueh, J. S. Yang, C. C. Lu, J. H. Chiang, M. Meng and J. G. Chung, Oncol. Rep., 2011, 26(1), 177–184 CAS.
- V. Ravikanth, P. S. Rana, L. Zhengjie, A. T. John, R. Agarwal and C. Agarwal, Carcinogenesis, 2006, 27, 1445–1453 CrossRef PubMed.
- C. Agarwal, T. Alpna and R. Agarwal, Mol. Cancer Ther., 2006, 5(12), 3294–3302 CrossRef CAS PubMed.
- C. Agarwal, R. Veluri, M. Kaur, S. C. Chou, J. A. Thompson and R. Agarwal, Carcinogenesis, 2007, 28(7), 1478–1484 CrossRef CAS PubMed.
- R. Komal, R. Subapriya, D. Gagan, S. Meenakshi, R. Agarwal and C. Agarwal, Mol. Cancer Ther., 2008, 7(5), 1258–1267 CrossRef PubMed.
- M. Kaur, B. Velmurugan, S. Rajamanickam, R. Agarwal and C. Agarwal, Pharm. Res., 2009, 26(9), 2133–2140 CrossRef CAS PubMed.
- S. Manato, K. Miyuki, T. Kazuya, M. Kiriko, K. Koichiro, K. Sei-ichi, H. Yasuna, F. Hiroshi and M. Hidefumi, Bioorg. Med. Chem. Lett., 2013, 23, 4935–4939 CrossRef PubMed.
- M. H. Li, M. Y. Wang, F. M. Zhao, H. B. Chen, H. G. Zhou, Q. C. Zhao, W. T. Li and M. H. Wu, Chin. Pharmacol. Bull., 2014, 30(5), 657–661 CAS.
- I. Makoto, S. Rie, S. Nahoko, L. Zong, T. Tadahira, O. Yokui, Y. J. Bao and C. Yingji, Biol. Pharm. Bull., 1995, 18(11), 1526–1530 Search PubMed.
- T. Ohno, M. Inoue and Y. Ogihara, Anticancer Res., 2001, 21(6A), 3875–3880 CAS.
- C. Lo, T. Y. Lai, J. H. Yang, J. S. Yang, Y. S. Ma, S. W. Weng, Y. Y. Chen, J. G. Lin and J. G. Chung, Int. J. Oncol., 2010, 37(2), 377–385 CAS.
- C. Lo, T. Y. Lai, J. S. Yang, J. H. Yang, Y. S. Ma, S. W. Weng, H. Y. Lin, H. Y. Chen, J. G. Lin and J. G. Chung, Melanoma Res., 2011, 21(4), 267–273 CrossRef CAS PubMed.
- V. Subramanian, B. Venkatesan, A. Tumala and E. Vellaichamy, Food Chem. Toxicol., 2014, 66, 44–55 CrossRef CAS PubMed.
- C. Locatelli, P. C. Leal, R. A. Yunes, R. J. Nunes and T. B. Creczynski-Pasa, Chem.-Biol. Interact., 2009, 181(2), 175–184 CrossRef CAS PubMed.
- E. Ortega, M. C. Sadaba, A. I. Ortiz, C. Cespon, A. Rocamora, J. M. Escolano, G. Roy, L. M. Villar and P. P. Gonzalez, Br. J. Cancer, 2003, 88(6), 940–943 CrossRef CAS PubMed.
- K. Yoshioka, T. Kataoka, T. Hayashi, M. Hasegawa, Y. Ishi and H. Hibasami, Oncol. Rep., 2000, 7(6), 1221–1223 CAS.
- P. D. Yumnam, U. Addepally, L. N. Mangamoori and K. Chepuri, IMPACT : International Journal of Research in Applied, Natural and Social Sciences, 2014, 2(5), 269–272 Search PubMed.
- K. Hamid, A. A. Abeer, A. Y. Wagee, M. A. Hapipah, A. A. Mahmood and H. Pouya, Arch. Pharm. Chem. Life Sci., 2011, 344, 703–709 CrossRef PubMed.
- N. S. Kim, S. I. Jeong, B. S. Hwang, Y. E. Lee, S. H. Kang, H. C. Lee and C. H. Oh, J. Med. Food, 2011, 14(3), 240–246 CrossRef CAS PubMed.
- A. Serrano, C. Palacios, G. Roy, C. Cespón, M. L. Villar, M. Nocito and P. P. González, Arch. Biochem. Biophys., 1998, 350(1), 49–54 CrossRef CAS.
- G. Roy, M. Lombardía, C. Palacios, A. Serrano, C. Cespón, E. Ortega, P. Eiras, S. Lujan, Y. Revilla and P. Gonzalez-Porqué, Arch. Biochem. Biophys., 2000, 383(2), 206–214 CrossRef CAS.
- S. Madlener, C. Illmer, Z. Horvath, P. Saiko, A. Losert, I. Herbacek, M. Grusch, H. L. Elford, G. Krupitza, A. Bernhaus, M. Fritzer-Szekeres and T. Szekeres, Cancer Lett., 2007, 245(1–2), 156–162 CrossRef CAS PubMed.
- R. D. Yeh, J. C. Chen, T. Y. Lai, J. S. Yang, C. S. Yu, J. H. Chiang, C. C. Lu, S. T. Yang, C. C. Yu, S. J. Chang, H. Y. Lin and J. G. Chung, Anticancer Res., 2011, 31(9), 2821–2832 CAS.
- M. Inoue, N. Sakaguchi, K. Isuzugawa, H. Tani and Y. Ogihara, Biol. Pharm. Bull., 2000, 23(10), 1153–1157 CAS.
- T. C. Reddy, D. B. Reddy, A. Aparna, K. M. Arunasree, G. Gupta, C. Achari, G. V. Reddy, V. Lakshmipathi, A. Subramanyam and P. Reddanna, Toxicol. In Vitro, 2012, 26(3), 396–405 CrossRef PubMed.
- C. Locatelli, R. Rosso, M. C. Santos-Silva, C. A. de Souza, M. A. Licínio, P. Leal, M. L. Bazzo and R. A. Yunes, Bioorg. Med. Chem., 2008, 16(7), 3791–3799 CrossRef CAS PubMed.
- H. Hla, R. Tsubouchi, M. Haneda, K. Murakami and M. Yoshino, Biomed. Res., 2002, 23, 127–134 CrossRef.
- I. Kazuto, I. Makoto and O. Yukio, Biol. Pharm. Bull., 2001, 24(7), 844–847 Search PubMed.
- M. López-Lázaro, J. M. Calderón-Montaño, E. Burgos-Morón and C. A. Austin, Mutagenesis, 2011, 26(4), 489–498 CrossRef PubMed.
- C. L. Teng, S. M. Han, W. C. Wu, C. M. Hsueh, J. R. Tsai, W. L. Hwang and S. L. Hsu, Food Chem. Toxicol., 2014, 71, 197–206 CrossRef CAS PubMed.
- C. H. Chen, W. C. Lin, C. N. Kuo and F. J. Lu, Food Chem. Toxicol., 2011, 49(2), 494–501 CrossRef CAS PubMed.
- A. Faried, D. kurnia, L. S. Faried, N. Usman, T. Miyazaki, H. Kato and H. Kuwano, Int. J. Oncol., 2007, 30, 605–613 CAS.
- Y. Gao, W. Li, L. Jia, B. Li, Y. C. Chen and Y. Tu, Biochem. Biophys. Res. Commun., 2013, 438(2), 370–374 CrossRef CAS PubMed.
- Z. Hou, S. Sang, H. You, M. J. Lee, J. Hong, K. V. Chin and C. S. Yang, Cancer Res., 2005, 65(17), 8049–8056 CAS.
- B. R. You, J. H. Moon, H. Y. Han and W. H. Park, Food Chem. Toxicol., 2010, 48, 1334–1340 CrossRef CAS PubMed.
- B. R. You and W. H. Park, J. Agric. Food Chem., 2011, 59(2), 763–771 CrossRef CAS PubMed.
- Z. Bing and H. U. Mengcai, Oncol. Lett., 2013, 6(6), 1749–1755 Search PubMed.
- M. Singh, K. Bhui, R. Singh and Y. Shukla, Life Sci., 2013, 93(1), 7–16 CrossRef CAS PubMed.
- Y. H. Han and W. H. Park, Food Chem. Toxicol., 2009, 47(10), 2531–2538 CrossRef CAS PubMed.
- G. S. Alyssa, H. W. Jeffrey, E. Hannah, F. R. Esther, R. B. Ayelet, R. W. Jordana, L. Tova, L. Z. Harriet and B. Harvey, Oxid. Antioxid. Med. Sci., 2013, 2(4), 265–274 CrossRef.
- C. C. Yi, R. Ranjan, C. Colonya and H. C. Robert, Molecules, 2010, 15, 8377–8389 CrossRef PubMed.
- C. L. Kuo, K. C. Lai, Y. S. Ma, S. W. Weng, J. P. Lin and J. G. Chung, Oncol. Rep., 2014, 32(1), 355–361 CAS.
- L. Cheng-zhen, Z. Xin, L. Hao, T. Yi-qing, T. Li-jiang, Y. Zi-ru, Z. Xiao-peng, S. Zhong-li and T. Hui-min, Cancer Biother.Radiopharm., 2012, 27(10), 701–710 CrossRef PubMed.
- C. L. Liao, K. C. Lai, A. C. Huang, J. S. Yang, J. J. Lin, S. H. Wu, W. W. Gibson, J. G. Lin and J. G. Chung, Food Chem. Toxicol., 2012, 50(5), 1734–1740 CrossRef CAS PubMed.
- J. D. Hsu, S. H. Kao, T. T. Ou, Y. J. Chen, Y. J. Li and C. J. Wang, J. Agric. Food Chem., 2011, 59(5), 1996–2003 CrossRef CAS PubMed.
- C. Annarica, M. José, M. Garcĺa, G. Lorena, J. T. Mercedes, T. Marĺa, O. Agulló, C. Pasqualina, L. R. Abelardo, A. Giuseppe, G. P. Pedro and M. P. Jorge, Carcinogenesis, 2006, 27(8), 1699–1712 Search PubMed.
- K. K. Min, J. K. Nam, J. J. Young, W. L. Ki and J. L. Hyong, Ann. N. Y. Acad. Sci., 2009, 1171, 514–520 CrossRef PubMed.
- H. H. Ho, C. S. Chang, W. S. Ho, S. Y. Liao, C. H. Wu and C. J. Wang, Food Chem. Toxicol., 2010, 48(8–9), 2508–2516 CrossRef CAS PubMed.
- T. T. Ou, C. J. Wang, Y. S. Lee, C. H. Wu and H. J. Lee, Mol. Nutr. Food Res., 2010, 54(12), 1781–1790 CAS.
- Z. Liu, D. Li, L. Yu and F. Niu, Chemotherapy, 2012, 58(3), 185–194 CrossRef CAS PubMed.
- M. Hirose, H. Yada, K. Hakoi, S. Takahashi and N. Ito, Carcinogenesis, 1993, 14(11), 2359–2364 CrossRef CAS PubMed.
- T. A. Martin, Y. Lin, A. J. Sanders, J. Lane and W. G. Jiang, Chapter: Cancer Invasion and Metastasis: Molecular and Cellular Perspective, in Metastatic Cancer: Clinical and Biological Perspectives, 2000 Search PubMed.
- Z. Liu, J. Schwimer, D. Liu, J. Lewis, F. L. Greenway, D. A. York and E. A. Woltering, Phytother. Res., 2006, 20(9), 806–813 CrossRef CAS PubMed.
- C. H. You, C. C. Ssu, H. L. Wen, Z. H. Dong, K. L. Ming, H. K. Yueh, T. W. Mei, J. C. Hsin and L. Y. Hsin, J. Ethnopharmacol., 2011, 134(1), 111–121 CrossRef PubMed.
- T. D. Gilmore, Oncogene, 2006, 25(51), 6680–6684 CrossRef CAS PubMed.
- A. P. J. Friedmann, M. Schneider, B. Proneth, Y. Y. Tyurina, V. A. Tyurin, V. J. Hammond, N. Herbach, M. Aichler, A. Walch, E. Eggenhofer, D. Basavarajappa, O. Rådmark, S. Kobayashi, T. Seibt, H. Beck, F. Neff, I. Esposito, R. Wanke, H. Förster, O. Yefremova, M. Heinrichmeyer, G. W. Bornkamm, E. K. Geissler, S. B. Thomas, B. R. Stockwell, V. B. O'Donnell, V. E. Kagan, J. A. Schick and M. Conrad, Nat. Cell Biol., 2014, 16(12), 1180–1191 CrossRef PubMed.
- R. Mathew, V. Karantza-Wadsworth and E. White, Nat. Rev. Cancer, 2007, 7(12), 961–967 CrossRef CAS PubMed.
- C. Peracchio, O. Alabiso, G. Valente and C. Isidoro, J. Ovarian Res., 2012, 5(1), 22 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |