Studies towards the total synthesis of solanoeclepin A: synthesis and potato cyst nematode hatching activity of analogues containing the tetracyclic left-hand substructure†
Abstract
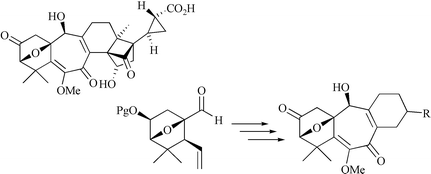
In our studies towards the total synthesis of solanoeclepin A, a natural hatching agent of potato cyst nematodes, three analogues containing the tetracyclic left-handed substructure have been synthesised. First, the synthesis of the parent tetracycle 2 in enantiopure form is reported. Key steps are (1) chromium-mediated coupling of aldehyde 5 (see preceding paper in this issue) and vinyl triflate 6 to furnish an α,β-unsaturated lactone, which was transformed into triene 4 in six-steps, (2) ring-closing metathesis of 4 to tetracycle 3 and (3) oxidative functionalisation of the least substituted double bond of 3 to provide the fully functionalised tetracyclic left-handed substructure of solanoeclepin A. The methodology developed was successfully applied in the synthesis of two more elaborate solanoeclepin A analogues 9 and 11. Both compounds, prepared as mixtures of diastereomers, showed promising biological activity in hatching activity tests.

 Please wait while we load your content...
Please wait while we load your content...