An unprecedented C80 cage that violates the isolated pentagon rule†
Pengwei
Yu‡
a,
Mengyang
Li‡
b,
Wangqiang
Shen
a,
Shuaifeng
Hu
a,
Peng-Yuan
Yu
a,
Xinyue
Tian
a,
Xiang
Zhao
 *b,
Lipiao
Bao
*b,
Lipiao
Bao
 *a and
Xing
Lu
*a and
Xing
Lu
 *a
*a
aState Key Laboratory of Materials Processing and Die & Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, 430074 China. E-mail: baol@hust.edu.cn; lux@hust.edu.cn
bInstitute of Molecular Science & Applied Chemistry, School of Chemistry, Xi'an Jiaotong University, Xi'an 710049, China. E-mail: xzhao@mail.xjtu.edu.cn
First published on 25th March 2022
Abstract
Two Lu2O@C80 isomers have been successfully isolated and unambiguously assigned as Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80, respectively, by X-ray crystallography. Interestingly, C1(31876)-C80 is an unprecedented cage with a pair of adjacent pentagons, which can be closely connected with C2v(5)-C80via a two-step Stone–Wales transformation. More importantly, the C1(31876)-C80 cage is a key point in the transformation map of oxide cluster fullerenes, filling the vacancy in the formation process.
Introduction
Endohedral metallofullerenes (EMFs) are a collection of novel molecules formed by the encapsulation of metal ions or clusters in fullerene cages.1,2 One of the most prominent features of these compounds is the charge transfer from the inner metallic species to the outer cages, thus stabilizing many cages that are unstable as empty fullerenes.3 For example, D5d(1)-C80 and D2(2)-C80 were separated as empty cages, but the encapsulation of M3N clusters accompanied by 6-electron transfer (M = Lu, Dy) promoted the formation of D5h(6)-C80 and Ih(7)-C80.4–8 In particular, the pristine cages of metallofullerenes can violate the isolated pentagon rule (IPR) that all empty fullerenes must obey because local steric strain on the adjacent pentagons (pentalene unit) can be released through interaction with the encapsulated metal.9–12 Since the first isolation of Sc2@C2v(4348)-C66 and Sc3N@D3(6140)-C68 in 2000, endohedrals that do not obey the IPR have received wide attention due to their unusual structures.13,14The formation mechanism of fullerenes remains unclear and controversial because their formation process cannot yet be directly observed. Fortunately, comprehensive inter-cage transformation pathways with cages containing pentalene units as key points are conducive to understanding the rearrangement process of fullerenes.15 Dorn et al. have shown that the unique asymmetric cage of M2C2@C1(51383)-C84 (M = Y, Gd) is a “missing link” in well-established conversion pathways to form many high-symmetry fullerene cages, which provides structural evidence for the top-down mechanism.16 Meanwhile, the bottom-up mechanism has also been well developed; Echegoyen et al. suggested that the nonclassical Sc2C2@Cs(hept)-C88 with a heptagon could be obtained from Sc2C2@C2v(9)-C86 through a simple C2 insertion.17 Recently, the interconversions between all isolated uranium-based mono-metallofullerenes reported by Chen et al. demonstrated that some cages with a pentalene unit can serve as precursors to form larger or smaller cages, indicating the simultaneous top-down and bottom-up processes.18,19 Accordingly, disclosing new cages that violate the isolated pentagon rule is crucial for completing the transformation map and revealing the formation mechanism of fullerenes.
Herein, we report the synthesis and isolation of two C80 isomers containing a Lu2O cluster. X-ray crystallographic results unambiguously reveal their molecular structures as Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80, respectively. It is rather surprising that a Lu2O@C1(31876)-C80 isomer with a pair of adjacent pentagons has a relatively high yield, and its cage can be interconverted to the C2v(5)-C80 cage through a two-step Stone–Wales transformation. Importantly, with the discovery of the unique C1(31876)-C80, a transformation map including the major cages of the reported dimetallic oxide cluster fullerenes is completed.
Results and discussion
Lutetium-based metallofullerenes were synthesized in an arc-discharge reactor under a He/CO2 atmosphere (270/15 Torr).20 After ultrasonic extraction in carbon disulfide (CS2), multistage high-performance liquid chromatography (HPLC) separation gave pure Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80 samples (Fig. S1†). The analytical HPLC chromatograms and the laser-desorption ionization time-of-flight (LDI-TOF) mass spectra confirmed their high purity (Fig. S2†).Absorption measurements were carried out for the two Lu2O@C80 isomers (Fig. 1a), which present obviously different characteristic absorptions in CS2. In detail, the Lu2O@C1(31876)-C80 isomer shows seven absorption peaks at 531, 592, 640, 834, 882, 940, and 1376 nm, whereas for Lu2O@C2v(5)-C80, six absorption peaks at 470, 618, 651, 688, 834, and 1399 nm are observed. Meanwhile, the absorption onsets of Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80 are around 1459 and 1600 nm, corresponding to small optical band gaps of 0.85 and 0.78 eV, respectively. The absorption features of Lu2O@C2v(5)-C80 resemble those of Sc2O@C2v(5)-C80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 and Sc2C2@C2v(5)-C80,22 indicating a four-electron transfer from the Lu2O cluster to the cage.
21 and Sc2C2@C2v(5)-C80,22 indicating a four-electron transfer from the Lu2O cluster to the cage.
The electrochemical properties of the Lu2O@C80 isomers were studied by cyclic voltammetry (Fig. 1b) and their redox potentials are summarized in Table 1 along with those of Sc2O@C2v(5)-C80. In the anodic region, both Lu2O@C80 isomers exhibit two fully reversible oxidation processes. The first and second oxidation potentials of Lu2O@C1(31876)-C80 are very close to the corresponding potentials of Lu2O@C2v(5)-C80. On the other hand, in the cathodic region, four and five reversible reduction processes are observed for Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80, respectively. The first reduction potential of Lu2O@C1(31876)-C80 shifts by −240 mV relative to that of Lu2O@C2v(5)-C80. Consequently, the electrochemical gap of Lu2O@C1(31876)-C80 (1.14 V) is larger than that of Lu2O@C2v(5)-C80 (0.95 V), which is in line with the absorption results. These results indicate that the difference in the cage structure has a considerable effect on the electrochemical behavior of the two molecules, especially on their lowest unoccupied molecular orbital energy, which is also reflected in the frontier molecular orbital analysis (Fig. S5†). Moreover, the redox behavior of Lu2O@C2v(5)-C80 resembles that of Sc2O@C2v(5)-C80,21 indicating their similar electronic structure. Thus, it can be concluded that the outer cage structure instead of the metallic unit has important influences on the electrochemical behavior of dimetallic oxide cluster fullerenes.
| Species | ox E 2 | ox E 1 | red E 1 | red E 2 | red E 3 | red E 4 | red E 5 | ΔEgap![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Half-wave potential in volts (reversible redox process). b Peak potential in volts (irreversible redox process). c ΔEgap = oxE1 − redE1. | |||||||||
| Lu2O@C1(31876)-C80 | 0.53a | 0.15a | −0.99a | −1.38a | −2.05a | −2.36a | 1.14 | This work | |
| Lu2O@C2v(5)-C80 | 0.56a | 0.20a | −0.75a | −1.12a | −1.44a | −1.78a | −2.19a | 0.95 | This work |
| Sc2O@C2v(5)-C80 | 0.56a | 0.24a | −0.89b | −1.48b | −1.75b | −1.96b | −2.13b | 1.13 | 20 |
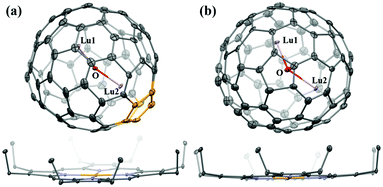
High-quality co-crystals of the two Lu2O@C80 isomers and NiII(OEP) (OEP = 2,3,7,8,12,13,17,18-octaethylporphin dianion) were obtained by layering a benzene solution of NiII(OEP) over a CS2 solution of each endohedral. Their molecular structures were unambiguously determined by single-crystal X-ray diffraction (XRD) crystallography as Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80, respectively. In particular, this is the first identification of the C1(31876)-C80 cage, which contains a pair of adjacent pentagons. The details of the crystallographic data are listed in Table S1.† The refined structures of Lu2O@C1(31876)-C80·NiII(OEP) and Lu2O@C2v(5)-C80·NiII(OEP) with the major metal sites are shown in Fig. 2. The porphyrin moiety leans towards the relatively flat region of each fullerene cage with the shortest Ni-to-cage-carbon distance of 2.959 Å and 2.899 Å for Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80, respectively, indicating substantial π–π interactions between the cluster fullerenes and NiII(OEP) molecules.23 Inside the two fullerene cages, the central oxygen atoms are both fully ordered, but the Lu atoms exhibit a certain degree of disorder (Fig. S3 and Table S2†), implying the motional behavior of the Lu atoms. Interestingly, it was found that one Lu atom prefers to vibrate near the adjacent pentagons in Lu2O@C1(31876)-C80, which is similar to the motion of metal atoms in the IPR-violating Dy2O@C2(13333)-C74.24 This phenomenon implies that the stabilization of cages that violate the isolated pentagon rule requires sufficient metal–pentalene coordination interactions.25 For the major metal sites (Fig. S4 and Table S3†), the average distance between Lu2 and the pentalene motif in Lu2O@C1(31876)-C80 is 2.389 Å, shorter than the other metal–cage distances inside the two isomers (2.570 Å for Lu1 in Lu2O@C1(31876)-C80; 2.526 Å for Lu1 and 2.581 Å for Lu2 in Lu2O@C2v(5)-C80). The Lu1–O–Lu2 angle in Lu2O@C1(31876)-C80 is 157.05°, larger than the value observed in Lu2O@C2v(5)-C80 (141.80°). These phenomena suggest that the pentalene unit in Lu2O@C1(31876)-C80 could provide stronger metal–pentalene interactions and extra space through protruding the cage.26
A complete transformation map of fullerenes can provide reliable evidence for understanding the formation mechanism. Importantly, the unique C1(31876)-C80 cage under study is essential to complete a transformation map for the major cages of the reported dimetallic oxide cluster fullerenes. The transformation processes involving C1(31876)-C80 simply require at most two well-established steps, i.e., the Stone–Wales transformation (SWT) or C2 loss.27,28Fig. 3 depicts the transformation map related to the C1(31876)-C80 cage and the detailed path of each step is illustrated in the ESI (Fig. S6–S10†). Structural rearrangements are demonstrated among three isomeric C82 cages, namely Cs(6)-C82, C2v(9)-C82 and C3v(8)-C82, via merely one SWT. C1(31876)-C80 is obtainable through a C2-unit loss in the pentalene motif generated by a SWT on Cs(6)-C82. Then two successive SWTs on C1(31876)-C80 afford C2v(5)-C80. In addition, the transformation from C1(31876)-C80 to C2v(3)-C78 is straightforward via a C2-unit loss on the pentalene motif, and then a further SWT on C2v(3)-C78 produces D3h(5)-C78. Interestingly, it is evident that the formation process of dimetallic oxide cluster fullerenes is different from that of well-studied monometallic actinide metallofullerenes, in which IPR-violating C1(17418)-C76 and C1(28324)-C80 cages are considered as the key intermediates.19,29 Thus, the present results also corroborate that endohedral metallic species affect the choice of the transformation pathways.16
 | ||
| Fig. 3 Fullerene transformation map related to the C1(31876)-C80 cage. Colors are used to visualize the motifs involved in the steps indicated by matching colored arrows. | ||
Theoretical calculations were performed at the PBE/6-31G(d)∼SDD level to investigate the origin of stability of the two Lu2O@C80 isomers. Fig. 4a and Table S4† show the relative energy and the energy gaps between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of Lu2O@C80 isomers. Although Lu2O@Ih(7)-C80 and Lu2O@D5h(6)-C80 possess the lowest energy of 0.0 and 3.4 kcal mol−1, respectively, their HOMO–LUMO gaps are too small (0.13 eV and 0.20 eV for Lu2O@Ih(7)-C80 and Lu2O@D5h(6)-C80, respectively), which means that the electrons on the HOMOs of Lu2O@Ih(7)-C80 and Lu2O@D5h(6)-C80 are easily excited, and thus these two isomers show high reactivity. On the basis of relative energy, Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80 are thermodynamic candidates with large HOMO–LUMO gaps. To verify this prediction, statistic thermodynamic analysis (Fig. 4b) considering the entropy–enthalpy effect, which has been verified as an effective method to determine the stable isomers of metallofullerenes in theory, such as dimetallic oxide cluster fullerene Sc2O@C78,30,31 was carried out. It is noteworthy that fullerenes are formed at very high temperatures (1500–3000 K) in an arc-discharge chamber. As shown in Fig. 4b, except for the highly reactive Lu2O@Ih(7)-C80 and Lu2O@D5h(6)-C80 isomers, the two Lu2O@C80 isomers under study, namely Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80, are the dominant species at 1500–3000 K. Consequently, the more competitive Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80 are obtained in the experiment instead of other isomers.
 | ||
| Fig. 4 (a) Relative energy (ΔE/kcal mol−1) and the energy gaps (Gap/eV) between the HOMO and the LUMO, and (b) relative concentration (ω/%) at different temperatures (T/K) of Lu2O@C80 isomers on the PBE/6-31G(d)-SDD. Isomers are numbered according to the spiral algorithm of Fowler and Manolopoulos.8 The simplified number is used for the IPR isomers. | ||
Conclusions
In summary, two isomers of Lu2O@C80, namely Lu2O@C1(31876)-C80 and Lu2O@C2v(5)-C80, have been successfully isolated and fully characterized by mass spectrometry, Vis-NIR absorption spectroscopy, cyclic voltammetry, single-crystal X-ray diffraction, and density functional theory calculations. Notably, this is the first observation of the C1(31876)-C80 fullerene cage, which contains a pair of adjacent pentagons. Further structural study reveals that the C1(31876)-C80 cage can serve as a key point in the transformation map for dimetallic oxide cluster fullerenes, filling the gap in the existing interconversion process. The current work demonstrates that the unique cage with adjacent pentagons may play a key role in the understanding of the long-standing puzzle about the formation mechanism and stimulates further interest in the exploration of more intermediates in the formation process.Experimental
Materials
Lutetium(III) oxide (Lu2O3, 99.999%) was purchased from Suzhou Lanxi New Material Co., Ltd. Graphite rods (∅ 8 × 115 mm, 99.999%) and graphite powder (320 mesh, 99.999%) were purchased from Shanghai Fengyi Carbon Co., Ltd. Carbon disulfide (CS2, 99.9%) was purchased from Aladdin company. Toluene (99.5%) was purchased from Sinopharm Chemical Reagent Co., Ltd. Chemicals were used as-received if not stated otherwise.Preparation and isolation
A core-drilled graphite rod (6.88 g) was filled with a homogeneous mixture of Lu2O3 (4.26 g) and graphite powder (2.19 g). The rods were annealed in a tube furnace at 1000 °C for 12 hours under an argon atmosphere and then vaporized in a Krätschmer–Huffman-type fullerene generator with an arc current of 100 A under a mixture atmosphere of 270 Torr helium and 15 Torr CO2. The as-produced fullerene soot was collected and sonicated in carbon disulfide for 1 h. After filtration, CS2 was removed using a rotary evaporator. The solid residue was dissolved in toluene and filtered. The isolation of Lu-based metallofullerenes was achieved by a multiple-stage HPLC process conducted on an LC-918 machine (Japan Analytical Industry Co. Ltd). In these processes, a Buckyprep column (∅ 20 × 250 mm), a Buckyprep-M column (∅ 20 × 250 mm), and a 5PBB column (∅ 20 × 250 mm) (all Cosmosil, Nacalai Tesque, Japan) were used. More details about the HPLC processes are described in the ESI.† In this study, 300 rods were used to obtain the desired amount of the two metallofullerene samples.Spectroscopic and electrochemical studies
LDI-TOF mass spectrometry was performed on a BIFLEX III spectrometer (Bruker Daltonics Inc., Germany). Vis-NIR spectra were obtained from a Lambda 35 spectrophotometer (PerkinElmer, USA) in CS2. CV curves were obtained in o-dichlorobenzene with 0.05 M TBAPF6 as the electrolyte using a CHI-660E instrument.Crystallographic characterization
Black co-crystals of the metallofullerenes and NiII(OEP) were obtained by layering a benzene solution of NiII(OEP) over a CS2 solution of the respective endohedrals in a glass tube at 0 °C for 30 days. Single-crystal X-ray data were collected at 100 K using a radiation wavelength of 0.73360 Å with a MarCCD detector at beamline BL17B in the Shanghai Synchrotron Radiation Facility. A multi-scan method was used for absorption corrections. The structures were solved by direct methods and refined with SHELXL-2014.32 Hydrogen atoms were inserted at the calculated positions and constrained with isotropic thermal parameters. The details of crystal data are listed in the ESI.†Computational studies
The optimizations of Lu2O@C80 isomers were carried out at the PBE/6-31G(d)∼SDD level without any imaginary frequency,33–36 where 6-31G(d) was for carbon and oxygen atoms and SDD with pseudopotentials was for lutetium atoms. PBE has been previously proved as a suitable functional for lutetium-based metallofullerenes.37 Statistic thermodynamic analysis including the entropy–enthalpy effect was carried out to determine the thermodynamically stable Lu2O@C80 isomers based on PBE/6-31G(d)∼SDD. Furthermore, single point calculations were conducted for thermodynamically stable Lu2O@C80 isomers on PBE/6-311G(d,p)∼def2TZVP, where 6-311G(d,p) was for carbon and oxygen atoms and the def2TZVP basis set with a small-core relativistic pseudopotential (14s13p10d8f6g)/[6s6p5d4f3g] was for lutetium atoms.38–41 All of the above calculations were performed with Gaussian 16 software except for the specific illustration.42Author contributions
X. Lu, L. Bao and P. Yu designed the research. P. Yu carried out the experiments. M. Li carried out the calculations. P. Yu, M. Li, S. Hu, P.-Y. Yu, X. Tian and W. Shen carried out the analysis. X. Lu and X. Zhao supervised the project. P. Yu and M. Li co-wrote the paper. X. Lu, L. Bao and X. Zhao guided and revised the paper. All authors read and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from NSFC (No. 21925104, 21573172, and 21773181) and the Hubei Provincial Natural Science Foundation of China (No. 2021CFA020) is gratefully acknowledged. We thank the staff at the BL17B beamline of the National Center for Protein Sciences Shanghai (NCPSS) at Shanghai Synchrotron Radiation Facility for the assistance with data collection. We thank the Analytical and Testing Center in Huazhong University of Science and Technology for all related measurements. X. Z. acknowledges financial support from the Nanotechnology Platform Program (Molecule and Material Synthesis) of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.References
- S. Yang, T. Wei and F. Jin, When metal clusters meet carbon cages: endohedral clusterfullerenes, Chem. Soc. Rev., 2017, 46, 5005–5058 RSC.
- W. Shen, S. Hu and X. Lu, Endohedral Metallofullerenes: New Structures and Unseen Phenomena, Chem. – Eur. J., 2020, 26, 5748–5757 CrossRef CAS PubMed.
- R. Guan, M. Chen, F. Jin and S. Yang, Strain Release of Fused Pentagons in Fullerene Cages by Chemical Functionalization, Angew. Chem., Int. Ed., 2020, 59, 1048–1073 CrossRef CAS PubMed.
- F. H. Hennrich, R. H. Michel, A. Fischer, S. Richard-Schneider, S. Gilb, M. M. Kappes, D. Fuchs, M. Bürk, K. Kobayashi and S. Nagase, Isolation and Characterization of C80, Angew. Chem., Int. Ed. Engl., 1996, 35, 1732–1734 CrossRef CAS.
- C. Wang, T. Sugai, T. Kai, T. Tomiyama and H. Shinohara, Production and isolation of an ellipsoidal C80 fullerene, Chem. Commun., 2000, 557–558, 10.1039/B000387P.
- W. Shen, L. Bao, S. Hu, X. Gao, Y. Xie, X. Gao, W. Huang and X. Lu, Isolation and Crystallographic Characterization of Lu3N@C2n, (2n = 80-88): Cage Selection by Cluster Size, Chem. – Eur. J., 2018, 24, 16692–16698 CrossRef CAS PubMed.
- S. Yang and L. Dunsch, Expanding the Number of Stable Isomeric Structures of the C80 Cage: A New Fullerene Dy3N@C80, Chem. – Eur. J., 2006, 12, 413–419 CrossRef PubMed.
- P. Fowler and D. Manolopoulos, An Atlas of Fullerenes, Oxford Press, Clarendon, 1995 Search PubMed.
- M. Guo, X. Li, Y.-R. Yao, J. Zhuang, Q. Meng, Y. Yan, X. Liu and N. Chen, A non-isolated pentagon rule C82 cage stabilized by a stretched Sc3N cluster, Chem. Commun., 2021, 57, 4150–4153 RSC.
- A. Liu, M. Nie, Y. Hao, Y. Yang, T. Wang, Z. Slanina, H. Cong, L. Feng, C. Wang and F. Uhlik, Ho2O@C74: Ho2O Cluster Expands within a Small Non-IPR Fullerene Cage of C,2(13333)-C74, Inorg. Chem., 2019, 58, 4774–4781 CrossRef CAS PubMed.
- S. Stevenson, A. J. Rothgeb, K. R. Tepper, J. Duchamp, H. C. Dorn, X. B. Powers, M. Roy, M. M. Olmstead and A. L. Balch, Isolation and Crystallographic Characterization of Two, Nonisolated Pentagon Endohedral Fullerenes: Ho3N@C,2(22010)-C78 and Tb3N@C2(22010)-C78, Chem. – Eur. J., 2019, 25, 12545–12551 CrossRef CAS PubMed.
- N. Chen, C. M. Beavers, M. Mulet-Gas, A. Rodríguez-Fortea, E. J. Munoz, Y. Li, M. M. Olmstead, A. L. Balch, J. M. Poblet and L. Echegoyen, Sc2S@Cs,(10528)-C72: A Dimetallic Sulfide Endohedral Fullerene with a Non Isolated Pentagon Rule Cage, J. Am. Chem. Soc., 2012, 134, 7851–7860 CrossRef CAS PubMed.
- C. Wang, T. Kai, T. Tomiyama, T. Yoshida, Y. Kobayashi, E. Nishibori, M. Takata, M. Sakata and H. Shinohara, C66 fullerene encaging a scandium dimer, Nature, 2000, 408, 426–427 CrossRef CAS PubMed.
- S. Stevenson, P. W. Fowler, T. Heine, J. C. Duchamp, G. Rice, T. Glass, K. Harich, E. Hajdu, R. Bible and H. C. Dorn, A stable non-classical metallofullerene family, Nature, 2000, 408, 427–428 CrossRef CAS PubMed.
- L. Bao, P. Yu, C. Pan, W. Shen and X. Lu, Crystallographic identification of Eu@C2n, (2n = 88, 86 and 84): completing a transformation map for existing metallofullerenes, Chem. Sci., 2019, 10, 2153–2158 RSC.
- J. Zhang, F. L. Bowles, D. W. Bearden, W. K. Ray, T. Fuhrer, Y. Ye, C. Dixon, K. Harich, R. F. Helm, M. M. Olmstead, A. L. Balch and H. C. Dorn, A missing link in the transformation from asymmetric to symmetric metallofullerene cages implies a top-down fullerene formation mechanism, Nat. Chem., 2013, 5, 880–885 CrossRef CAS PubMed.
- C.-H. Chen, L. Abella, M. R. Cerón, M. A. Guerrero-Ayala, A. Rodríguez-Fortea, M. M. Olmstead, X. B. Powers, A. L. Balch, J. M. Poblet and L. Echegoyen, Zigzag Sc2C2 Carbide Cluster inside a [88]Fullerene Cage with One Heptagon, Sc2C2@Cs(hept)-C88: A Kinetically Trapped Fullerene Formed by C2 Insertion?, J. Am. Chem. Soc., 2016, 138, 13030–13037 CrossRef CAS PubMed.
- W. Cai, L. Abella, J. Zhuang, X. Zhang, L. Feng, Y. Wang, R. Morales-Martínez, R. Esper, M. Boero, A. Metta-Magaña, A. Rodríguez-Fortea, J. M. Poblet, L. Echegoyen and N. Chen, Synthesis and Characterization of Non-Isolated-Pentagon-Rule Actinide Endohedral Metallofullerenes U@C,1(17418)-C76, U@C1(28324)-C80, and Th@C1(28324)-C80: Low-Symmetry Cage Selection Directed by a Tetravalent Ion, J. Am. Chem. Soc., 2018, 140, 18039–18050 CrossRef CAS PubMed.
- W. Cai, J. Alvarado, A. Metta-Magaña, N. Chen and L. Echegoyen, Interconversions between Uranium Mono-metallofullerenes: Mechanistic Implications and Role of Asymmetric Cages, J. Am. Chem. Soc., 2020, 142, 13112–13119 CrossRef CAS PubMed.
- W. Krätschmer, K. Fostiropoulos and D. R. Huffman, The infrared and ultraviolet absorption spectra of laboratory-produced carbon dust: evidence for the presence of the C60 molecule, Chem. Phys. Lett., 1990, 170, 167–170 CrossRef.
- Q. Tang, L. Abella, Y. Hao, X. Li, Y. Wan, A. Rodríguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Sc2O@C2v(5)-C80: Dimetallic Oxide Cluster Inside a C80 Fullerene Cage, Inorg. Chem., 2015, 54, 9845–9852 CrossRef CAS PubMed.
- H. Kurihara, X. Lu, Y. Iiduka, N. Mizorogi, Z. Slanina, T. Tsuchiya, T. Akasaka and S. Nagase, Sc2C2@C80 Rather than Sc2@C82: Templated Formation of Unexpected C2v(5)-C80 and Temperature-Dependent Dynamic Motion of Internal Sc2C2 Cluster, J. Am. Chem. Soc., 2011, 133, 2382–2385 CrossRef CAS PubMed.
- L. N. Dawe, T. A. AlHujran, H.-A. Tran, J. I. Mercer, E. A. Jackson, L. T. Scott and P. E. Georghiou, Corannulene and its penta-tert-butyl derivative co-crystallize 1:1 with pristine C60-fullerene, Chem. Commun., 2012, 48, 5563–5565 RSC.
- G. Velkos, W. Yang, Y.-R. Yao, S. M. Sudarkova, X. Liu, B. Büchner, S. M. Avdoshenko, N. Chen and A. A. Popov, Shape-adaptive single-molecule magnetism and hysteresis up to 14 K in oxide clusterfullerenes Dy2O@C72 and Dy2O@C74 with fused pentagon pairs and flexible Dy–(μ2-O)–Dy angle, Chem. Sci., 2020, 11, 4766–4772 RSC.
- Y. Hao, L. Feng, W. Xu, Z. Gu, Z. Hu, Z. Shi, Z. Slanina and F. Uhlík, Sm@C2v(19138)-C76: A Non-IPR Cage Stabilized by a Divalent Metal Ion, Inorg. Chem., 2015, 54, 4243–4248 CrossRef CAS PubMed.
- C. M. Beavers, M. N. Chaur, M. M. Olmstead, L. Echegoyen and A. L. Balch, Large Metal Ions in a Relatively Small Fullerene Cage: The Structure of Gd3N@C,2(22010)-C78 Departs from the Isolated Pentagon Rule, J. Am. Chem. Soc., 2009, 131, 11519–11524 CrossRef CAS PubMed.
- A. J. Stone and D. J. Wales, Theoretical studies of icosahedral C60 and some related species, Chem. Phys. Lett., 1986, 128, 501–503 CrossRef CAS.
- D. J. Wales, M. A. Miller and T. R. Walsh, Archetypal energy landscapes, Nature, 1998, 394, 758–760 CrossRef CAS.
- W. Cai, C.-H. Chen, N. Chen and L. Echegoyen, Fullerenes as Nanocontainers That Stabilize Unique Actinide Species Inside: Structures, Formation, and Reactivity, Acc. Chem. Res., 2019, 52, 1824–1833 CrossRef CAS PubMed.
- P. Zhao, M. Li, Y.-J. Guo, R. Zhao and X. Zhao, Single Step Stone-Wales Transformation Linking Two Thermodynamically Stable Sc2O@C78 Isomers, Inorg. Chem., 2016, 55, 2220–2226 CrossRef CAS PubMed.
- Y. Hao, Q. Tang, X. Li, M. Zhang, Y. Wan, L. Feng, N. Chen, Z. Slanina, L. Adamowicz and F. Uhlík, Isomeric Sc2O@C78 Related by a Single-Step Stone-Wales Transformation: Key Links in an Unprecedented Fullerene Formation Pathway, Inorg. Chem., 2016, 55, 11354–11361 CrossRef CAS PubMed.
- G. Sheldrick, A short history of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- M. Ernzerhof and G. E. Scuseria, Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- W. J. Hehre, R. Ditchfield and J. A. Pople, Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS.
- P. C. Hariharan and J. A. Pople, The influence of polarization functions on molecular orbital hydrogenation energies, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- M. Dolg, H. Stoll and H. Preuss, A combination of quasirelativistic pseudopotential and ligand field calculations for lanthanoid compounds, Theor. Chim. Acta, 1993, 85, 441–450 CrossRef CAS.
- K. Zhang, H. Zheng, M. Li, Q. Li, Y. Zhao and X. Zhao, Significant Roles of a Particularly Stable Two-Center Two-Electron Lu–Lu σ Bond in Lu2@C86: Electronic Structure of Lu and Radius of Lu2+, Inorg. Chem., 2021, 60, 2425–2436 CrossRef CAS PubMed.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, Self–consistent molecular orbital methods. XX. A basis set for correlated wave functions, J. Chem. Phys., 1980, 72, 650–654 CrossRef CAS.
- X. Cao and M. Dolg, Valence basis sets for relativistic energy-consistent small-core lanthanide pseudopotentials, J. Chem. Phys., 2001, 115, 7348–7355 CrossRef CAS.
- R. Gulde, P. Pollak and F. Weigend, Error-Balanced Segmented Contracted Basis Sets of Double-ζ to Quadruple-ζ Valence Quality for the Lanthanides, J. Chem. Theory Comput., 2012, 8, 4062–4068 CrossRef CAS PubMed.
- B. P. Pritchard, D. Altarawy, B. Didier, T. D. Gibson and T. L. Windus, New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community, J. Chem. Inf. Model., 2019, 59, 4814–4820 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2131027 and 2131029. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi00410k |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2022 |