DOI:
10.1039/B105179M
(Paper)
J. Mater. Chem., 2002,
12, 148-152
Magnetic properties of silver lanthanide molybdates AgLnMo2O8
(Ln = lanthanide)
Received
13th June 2001
, Accepted 12th October 2001
First published on 27th November 2001
Abstract
The magnetic properties of scheelite related compounds AgLnMo2O8
(Ln = lanthanide) are reported. Magnetic susceptibility measurements show that the compounds are paramagnetic down to 2 K with AgSmMo2O8 and AgEuMo2O8 showing Van Vleck paramagnetism. A 151Eu Mössbauer spectrum for AgEuMo2O8 indicates that the Eu ion is in the trivalent state and that an axial electric field gradient exists in this compound. Both magnetic susceptibility and electron paramagnetic resonance (EPR) measurements show that the Gd3+ ion is in the 8S7/2 state.
Introduction
There are a considerable number of scheelite (CaWO4) related molybdates. The compositions of these compounds are represented as AMoO4 or A′A″Mo2O8, where A = alkaline earth ion, Pb2+ or Hg2+; A′ = alkali metal ion or Ag+; A″ = lanthanide ion or Bi3+. We are interested in the latter composition-type of compounds containing lanthanide elements, ALnMo2O8
(Ln = lanthanide ion). The preparation, crystal structures, and some physical properties of lanthanide molybdates containing silver, AgLnMo2O8, have been reported by several researchers.1–5 These compounds crystallize in the scheelite related structure as shown in Fig. 1.
Rath and Müller-Buschbaum elucidated that the crystal structures of AgSmMo2O8 and AgYbMo2O8 had tetragonal symmetry with space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) (no. 82), by the X-ray diffraction method for their single crystals. The crystal structures are closely related to the scheelite type, with Ag+ and Ln3+ ions in statistical distribution.3 Shi et al. reported that the temperature dependence of the magnetic susceptibilities for AgLnMo2O8
(Ln = Ce–Nd, Sm, Gd and Tb) reveals Curie–Weiss law behavior with three anomalies occurring at 100, 160 and 230 K, which are observed in all samples investigated in the temperature range studied (77 ≤ T ≤ 300 K).4,5
(no. 82), by the X-ray diffraction method for their single crystals. The crystal structures are closely related to the scheelite type, with Ag+ and Ln3+ ions in statistical distribution.3 Shi et al. reported that the temperature dependence of the magnetic susceptibilities for AgLnMo2O8
(Ln = Ce–Nd, Sm, Gd and Tb) reveals Curie–Weiss law behavior with three anomalies occurring at 100, 160 and 230 K, which are observed in all samples investigated in the temperature range studied (77 ≤ T ≤ 300 K).4,5
 |
| | Fig. 1
Crystal structure of AgLnMo2O8.
| |
In this study, we prepared a series of lanthanide molybdates with silver, AgLnMo2O8
(Ln = lanthanide) and determined their crystal structures by X-ray diffraction measurements. Magnetic susceptibilities were measured in the temperature range 2–300 K to investigate whether magnetic anomalies are observed and to elucidate the magnetic behavior at lower temperatures. In addition, electron paramagnetic resonance (EPR) and Mössbauer spectrum measurements have been performed.
Experimental
Sample preparation
As starting materials, Ag metal powder, rare earth oxides Ln2O3
(Ln = Y, La, Nd, Sm, Eu, Gd, Dy, Ho, Er, Tm, Yb, and Lu), CeO2, Pr6O11, Tb4O7, and molybdenum(VI) oxide MoO3
(all with purity >99.9%) were used. Before use, the rare earth oxides were heated in air at 1173 K to remove any moisture and oxidized to the stoichiometric compositions, i.e., Ln2O3.00, CeO2.00, Pr6O11.00 and Tb4O7.00. They were weighed in the appropriate metal ratios (Ag∶Ln∶Mo = 1∶1∶2), and intimately mixed in an agate mortar. The mixtures were pressed into pellets and then heated in air at 1073 K for 12 h. After cooling to room temperature, the pellets were crushed into a powder, re-ground, re-pressed
into pellets, and heated again at 1073 K for 12 h to complete the reaction.
Analysis
X-Ray powder diffraction measurements were performed with Cu-Kα radiation on a Rigaku MultiFlex diffractometer equipped with a curved graphite monochromator. Intensity data were collected by step scanning in the range 10–120° at intervals of 0.02°. The structure and lattice parameters were refined with a Rietveld program RIETAN 2000.6
Magnetic measurements
Magnetic susceptibility measurements were performed with a SQUID magnetometer (Quantum Design MPMS model). The temperature dependence of the magnetic susceptibilities was investigated under both zero-field-cooled condition (ZFC) and field-cooled condition (FC). The former was measured on heating the sample to 300 K after zero-field cooling to 2 K. The applied magnetic field was 1000 G. The latter was measured on cooling the sample from 300 to 2 K at 1000 G.
151Eu Mössbauer spectrum measurements
151Eu Mössbauer spectra were measured with a VT-6000 spectrometer (Laboratory Equipment Co., Japan) at room temperature. The absorber material AgEuMo2O8 was mixed with fine graphite powder and ground in an agate mortar. The average thickness of AgEuMo2O8 was 10 mg Eu cm−2. The 151Sm2O3 source emitting 21.6 keV γ-rays was maintained at room temperature. The spectrometer was operated in the velocity range ±10 mm s−1. The velocity scale was calibrated with the six-line magnetic hyperfine spectrum of α-Fe using a 57Co source. Zero velocity was taken as the absorption of 151Eu in crystalline EuF3.
Electron paramagnetic resonance measurements
The EPR spectra at X band (9.096 GHz) were measured using a JEOL RE–2X spectrometer. The magnetic field was swept from 100 to 8000 G, which was monitored with a proton NMR gaussmeter, and the microwave frequency was measured with a frequency counter. Before the samples were measured, a blank tube was recorded to eliminate the possibility of interference by the background resonance of the cavity and/or the sample tube.
Results and discussion
1. Crystal structures
The results of the X-ray diffraction measurements show that the desired tetragonal scheelite-type compounds AgLnMo2O8
(Ln = Y, La–Nd and Sm–Lu) with space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) could be prepared as single-phase materials. Fig. 2 shows the X-ray diffraction pattern for AgGdMo2O8, as an example. We have performed Rietveld analysis for the XRD data. Table 1 lists the lattice parameters for AgLnMo2O8. The lattice parameters for AgLnMo2O8 are in excellent agreement with those reported by other workers.2–5 It has been found that the silver and lanthanide atoms randomly occupy the 2b and 2d sites. The (1/2Ag + 1/2Ln)1, (1/2Ag + 1/2Ln)2,
Mo1, Mo2, O1 and O2 ions occupy the 2d(1/2,0,1/4), 2b(1/2,1/2,0), 2a(0,0,0), 2c(0,1/2,1/4), 8g(x,y,z), and 8g(x,y,z) sites, respectively. The crystallographic positional parameters for AgLnMo2O8 after refinement are listed in Table 1.
could be prepared as single-phase materials. Fig. 2 shows the X-ray diffraction pattern for AgGdMo2O8, as an example. We have performed Rietveld analysis for the XRD data. Table 1 lists the lattice parameters for AgLnMo2O8. The lattice parameters for AgLnMo2O8 are in excellent agreement with those reported by other workers.2–5 It has been found that the silver and lanthanide atoms randomly occupy the 2b and 2d sites. The (1/2Ag + 1/2Ln)1, (1/2Ag + 1/2Ln)2,
Mo1, Mo2, O1 and O2 ions occupy the 2d(1/2,0,1/4), 2b(1/2,1/2,0), 2a(0,0,0), 2c(0,1/2,1/4), 8g(x,y,z), and 8g(x,y,z) sites, respectively. The crystallographic positional parameters for AgLnMo2O8 after refinement are listed in Table 1.
 |
| | Fig. 2
Observed and calculated X-ray powder diffraction patterns for AgGdMo2O8. The observed data are indicated by crosses and the calculated pattern is the solid line. The short vertical lines below the patterns mark the positions of all possible Bragg reflections. The bottom continuous line is the difference between the observed and the calculated intensity.
| |
Table 1
Lattice parameters and crystallographic positional parameters for AgLnMo2O8
a
| |
Y |
La |
Ce |
Pr |
Nd |
Sm |
Eu |
Gd |
Tb |
Dy |
Ho |
Er |
Tm |
Yb |
Lu |
|
Note: (1/2Ag + 1/2Ln)1, (1/2Ag + 1/2Ln)2, Mo1, and Mo2 occupy 2d(1/2,0,1/4), 2b(1/2,1/2,0), 2a(0,0,0) and 2c(0,1/2,1/4) sites, respectively.
|
|
a/nm |
0.52139(2) |
0.53568(2) |
0.53348(2) |
0.53192(3) |
0.53021(1) |
0.52753(1) |
0.52629(2) |
0.52522(1) |
0.52385(1) |
0.52260(1) |
0.52153(1) |
0.52046(1) |
0.51952(2) |
0.51853(2) |
0.51744(2) |
|
c/nm |
1.14403(5) |
1.17527(4) |
1.16885(4) |
1.16529(8) |
1.16186(3) |
1.15593(2) |
1.15391(4) |
1.15175(3) |
1.14824(2) |
1.14593(3) |
1.14362(3) |
1.14179(3) |
1.13940(3) |
1.13754(4) |
1.13699(6) |
|
V/nm3 |
0.31100(2) |
0.33724(2) |
0.33266(2) |
0.32970(4) |
0.32662(1) |
0.32168(1) |
0.31961(2) |
0.31772(1) |
0.31509(1) |
0.31297(1) |
0.31105(1) |
0.30928(1) |
0.30752(2) |
0.30585(2) |
0.30442(2) |
| O(1)
x |
0.249(7) |
0.257(2) |
0.283(2) |
0.254(3) |
0.215(3) |
0.260(3) |
0.259(2) |
0.254(2) |
0.248(3) |
0.210(3) |
0.219(3) |
0.249(2) |
0.211(3) |
0.222(4) |
0.232(2) |
| O(1)
y |
0.151(7) |
0.122(4) |
0.086(3) |
0.150(1) |
0.177(4) |
0.144(5) |
0.135(5) |
0.141(4) |
0.142(5) |
0.154(6) |
0.127(3) |
0.158(3) |
0.113(3) |
0.125(4) |
0.127(6) |
| O(1)
z |
0.084(3) |
0.082(2) |
0.064(1) |
0.077(1) |
0.090(1) |
0.082(2) |
0.082(2) |
0.083(1) |
0.081(2) |
0.087(1) |
0.075(1) |
0.087(1) |
0.076(1) |
0.080(1) |
0.084(2) |
| O(2)
x |
0.241(7) |
0.207(3) |
0.224(3) |
0.223(1) |
0.245(3) |
0.218(3) |
0.206(3) |
0.198(3) |
0.211(3) |
0.247(2) |
0.245(3) |
0.216(2) |
0.253(3) |
0.249(4) |
0.247(4) |
| O(2)
y |
0.652(9) |
0.656(5) |
0.666(3) |
0.649(1) |
0.616(4) |
0.629(5) |
0.663(6) |
0.648(6) |
0.656(7) |
0.646(4) |
0.658(2) |
0.631(3) |
0.667(3) |
0.664(4) |
0.658(5) |
| O(2)
z |
0.163(5) |
0.170(2) |
0.169(1) |
0.161(1) |
0.177(1) |
0.169(3) |
0.165(2) |
0.164(2) |
0.165(2) |
0.170(1) |
0.162(1) |
0.172(1) |
0.162(1) |
0.161(1) |
0.162(1) |
2. Magnetic susceptibilities
The results of the magnetic susceptibility measurements for AgLnMo2O8 except for the diamagnetic Y, La and Lu compounds show that they are paramagnetic down to 2 K. No magnetic anomaly is detected for any AgLnMo2O8 in our investigation. The temperature dependences of the reciprocal magnetic susceptibilities for AgLnMo2O8 compounds are shown in Figs. 3 and 4. No difference between the ZFC magnetic susceptibilities and the FC magnetic susceptibilities is observed. The electronic configurations of Ag+ and Mo6+ are [Kr]4d10 and [Kr]4d0
([Kr]: krypton core), respectively, i.e., these ions are diamagnetic. Therefore, the only paramagnetic ion in AgLnMo2O8 is Ln3+. The susceptibilities, χM, for AgLnMo2O8
generally obey the Curie–Weiss law except for AgSmMo2O8 and AgEuMo2O8. Deviation from the Curie–Weiss law is, however, clearly seen in the susceptibilities for the Ce, Pr, Nd and Yb compounds. The reciprocal susceptibilities χ−1 of these compounds at low temperatures decrease rapidly with decreasing temperature except for the Pr compound. This deviation may originate from crystal field effects. The temperature dependence of the magnetic susceptibilities for the Sm and Eu compounds is characteristic of Van Vleck paramagnets, which will be discussed later. Table 2 shows the effective magnetic moments of these compounds, μexptl, and calculated moments for the free Ln3+ ions, μcalc. The effective magnetic moments obtained experimentally (μexptl) are in good agreement with
the calculated values (μcalc). In the following, we will discuss the magnetic behavior of the Ln3+ ions in each of these compounds.
 |
| | Fig. 3
Temperature dependence of reciprocal magnetic susceptibilities for AgLnMo2O8
(Ln = Ce, Pr, Nd, Sm and Eu).
| |
 |
| | Fig. 4
Temperature dependence of reciprocal magnetic susceptibilities for AgLnMo2O8
(Ln = Gd, Tb, Dy, Ho, Er, Tm and Yb).
| |
Table 2
Experimental magnetic moments of Ln3+
(μexptl) and calculated magnetic moments (μcalc) for AgLnMo2O8
| Ln3+ |
μ
exptl/μB |
μ
calc/μB |
| Ce3+ |
2.56 |
2.54 |
| Pr3+ |
3.58 |
3.58 |
| Nd3+ |
3.78 |
3.62 |
| Gd3+ |
7.92 |
7.94 |
| Tb3+ |
9.71 |
9.72 |
| Dy3+ |
10.54 |
10.63 |
| Ho3+ |
10.62 |
10.60 |
| Er3+ |
9.53 |
9.59 |
| Tm3+ |
7.62 |
7.57 |
| Yb3+ |
4.70 |
4.54 |
3. Magnetic properties of AgSmMo2O8
Fig. 5 shows the temperature dependence of the magnetic susceptibilities of the Sm3+ ion in AgSmMo2O8. This shows a large variation at lower temperatures and small variation at higher temperatures. This behavior can be explained using Van Vleck theory.7 The ground state of the Sm3+ ion is 6H5/2. At low temperatures, only the magnetic 6H5/2 ground multiplet is populated. However, since the energy difference between the ground state and the first excited state 6H7/2 is not so large compared to the thermal energy kBT
(kB is the Boltzmann constant), we cannot neglect the mixing effect in the calculation of the magnetic susceptibilities at higher temperatures. According to the Van Vleck theory, the molar magnetic susceptibility of the
Sm3+ ion is given by eqn. (1):| |  | (1) |
where NA and μB are the Avogadro number and the Bohr magneton, respectively. The parameter y = λ/kBT is 1/55 and corresponds to the ratio of the overall multiplet width (the spin–orbit coupling constant, λ) and the thermal energy (kBT). Although the fitting of eqn. (1) to the experimental data (solid line in Fig. 5) is not good at lower temperatures, the spin–orbit coupling constant λ is found to be
λ = 306 cm−1. Then, the energy difference between the ground state 6H5/2
and the first excited state 6H7/2 is determined to be 1071 cm−1, which is near to the theoretical value.7
 |
| | Fig. 5
Temperature dependence of the magnetic susceptibility of the Sm3+ ion in AgSmMo2O8; fitting with eqn. (1) is shown (solid line).
| |
4. Magnetic susceptibilities of AgEuMo2O8
Fig. 6 shows the magnetic susceptibility of the Eu3+ ion in AgEuMo2O8 as a function of temperature. Except at very low temperatures, the shape of this susceptibility vs. temperature curve is characteristic of Van Vleck paramagnetism, with a constant susceptibility for the lower temperature range and a decreasing susceptibility with increasing temperature for T ≥ 100 K. At very low temperatures (T ≤ 10 K), the magnetic susceptibilities increase suddenly with decreasing temperature. This behavior may be attributable to paramagnetic Mo5+ ions formed to a small extent due to the oxygen deficiency.
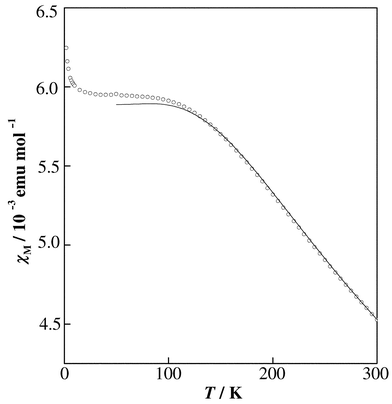 |
| | Fig. 6
Temperature dependence of the magnetic susceptibility of the Eu3+ ion in AgEuMo2O8; fitting with eqn. (2) is shown (solid line).
| |
The ground state 7F0 of Eu3+ is nonmagnetic and the excited state 7F1 is close enough to it to give an energy difference comparable to kBT at room temperature. Therefore, the magnetic susceptibility becomes independent of temperature at lower temperatures. The molar magnetic susceptibility of the Eu3+ ion is expressed by eqn. (2):7
| |  | (2) |
where
x =
λ/
kBT is 1/21 corresponding to the ratio of the overall multiplet width to
kBT. The magnetic susceptibilities calculated by using
eqn. (2) are fitted to the measured susceptibilities in the range 50–300 K, as shown in
Fig. 6
with a solid line. The spin–orbit coupling constant
λ of Eu
3+, which is the energy-level difference between the ground state
7F
0 and the first excited state
7F
1, is determined to be 354 cm
−1 for AgEuMo
2O
8. This value is close to the values reported in other complex
oxides, for example, 339 cm
−1
(Ba
2EuNbO
6)
8 and 364 cm
−1
(Ba
2EuIrO
6).
9
5.
151Eu Mössbauer spectrum of AgEuMo2O8
The 151Eu Mössbauer spectrum of AgEuMo2O8 measured at room temperature is shown in Fig. 7. The Eu ions in AgEuMo2O8 are located in two crystallographically nonequivalent sites, 2b and 2d. Both of them occupy fourfold symmetry sites (![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) symmetry), therefore, the electric field gradient (EFG) tensor at this site is axially symmetric (asymmetry parameter, η = 0). The hyperfine quadrupole interaction for this axial EFG can be written as shown in eqn. (3):
symmetry), therefore, the electric field gradient (EFG) tensor at this site is axially symmetric (asymmetry parameter, η = 0). The hyperfine quadrupole interaction for this axial EFG can be written as shown in eqn. (3):| |  | (3) |
where I is the nuclear spin, Q is the quadrupole moment, and eq = Vzz is the principal EFG. The eight allowed transitions due to a quadrupole interaction were taken into account. Fig. 8 shows eight possible transitions due to a quadrupole interaction. The observed spectrum in Fig. 7 was fitted with the sum of these Lorenzian lines. The fit assumes eight transition lines with equal linewidths. The positions of these lines are specified by the isomer shift δ and the quadrupole coupling constant e2qQ0. The intensity ratios of the eight lines are determined by the Clebsh–Gordan coefficients. The ratio of the excited and ground state quadrupole moments Qe/Q0 is taken to be 1.34.10 Since there are two nonequivalent Eu sites the Mössbauer spectrum should consist of 16 lines. We thus attempted to fit the spectrum with 16 transition lines. However, these lines were not completely resolved, indicating that the environments of the two Eu sites are quite similar. The line
width used to fit the AgEuMo2O8 spectrum and that for the reference EuF3 spectrum were 2.14(9) and 2.72(7) mm s−1, respectively. The average isomer shift δ for AgEuMo2O8 is 0.388(9) mm s−1, which confirms that the Eu ions are in the trivalent state. The average quadrupole coupling constant e2qQ0 is −3.07(49) mm s−1 and negative in this compound.
 |
| | Fig. 7
151Eu Mössbauer spectra of AgEuMo2O8. The solid line is a computed fit with eight Lorentzians.
| |
 |
| | Fig. 8
Eight transitions due to a quadrupole interaction of 151Eu.
| |
6. Magnetic properties of AgGdMo2O8
The temperature dependence of the reciprocal magnetic susceptibilities of the Gd3+ ion in AgGdMo2O8 is shown in Fig. 9. The reciprocal susceptibilities χ−1 are almost linear with temperature in the measured temperature range of 2–300 K. The effective magnetic moment of the Gd3+ ion is determined to be 7.92 μB, which is close to the theoretical value of 7.94 μB for the free Gd3+ ion. The result indicates the eight-fold ground state 8S7/2 is not split by the crystal field.
 |
| | Fig. 9
Temperature dependence of the magnetic susceptibility of Gd3+ ion in AgGdMo2O8; Curie–Weiss law fitting is shown by the solid line.
| |
Fig. 10 shows the X-band EPR spectrum for AgGdMo2O8 measured at room temperature. A single broad spectrum centered at a magnetic field of 3336 G was observed. The effective magnetic moment of the Gd3+ ion is calculated to be 8.02 μB from the EPR data (g = 2.02). The effective magnetic moments of the Gd3+ ion determined from the magnetic susceptibility and the EPR measurements are in good agreement with the calculated magnetic moment (7.94 μB), which indicates that the ground state of the Gd3+ ion in AgGdMo2O8 is a pure 8S7/2 state.
 |
| | Fig. 10
EPR spectrum of Gd3+ in AgGdMo2O8 at room temperature.
| |
In summary, the magnetic properties of AgLnMo2O8
(Ln = lanthanide element) have been investigated. Magnetic susceptibility measurements show that they are paramagnetic down to 2 K. 151Eu Mössbauer and EPR spectra measurements indicate that lanthanide ion is in the trivalent state for AgLnMo2O8.
References
- A. W. Sleight, K. Aykan and D. B. Rogers, J. Solid State Chem., 1975, 13, 231–236 CrossRef CAS.
- A. P. Perepelitsa, M. V. Artemenko and V. N. Ishchenko, Russ. J. Inorg. Chem., 1983, 28, 1123–1124 Search PubMed.
- M. Rath and Hk. Müller-Buschbaum, J. Alloys Compd., 1993, 198, 193–196 CrossRef CAS.
- F. Shi, J. Meng and Y. Ren, Mater. Res. Bull., 1995, 30, 1401–1405 CrossRef CAS.
- F. Shi, J. Meng and Y. Ren, J. Alloys Compd., 1996, 233, 56–60 CrossRef CAS.
- F. Izumi and T. Ikeda, Mater. Sci. Forum, 2000, 198, 321–324 Search PubMed.
-
J. H. Van Vleck, Theory of Electric and Magnetic Susceptibilities, Clarendon, Oxford, 1932 Search PubMed.
- K. Henmi, Y. Hinatsu and N. M. Masaki, J. Solid State Chem., 1999, 148, 353–360 CrossRef CAS.
- M. Wakeshima, D. Harada, Y. Hinatsu and N. Masaki, J.
Solid State Chem., 1999, 147, 618–623 CrossRef CAS.
- C. L. Chein, S. DeBenedetti and F. de S. Barros, Phys. Rev. B, 1974, 10, 3913–3922 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2002 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) (no. 82), by the X-ray diffraction method for their single crystals. The crystal structures are closely related to the scheelite type, with Ag+ and Ln3+ ions in statistical distribution.3 Shi et al. reported that the temperature dependence of the magnetic susceptibilities for AgLnMo2O8
(Ln = Ce–Nd, Sm, Gd and Tb) reveals Curie–Weiss law behavior with three anomalies occurring at 100, 160 and 230 K, which are observed in all samples investigated in the temperature range studied (77 ≤ T ≤ 300 K).4,5
(no. 82), by the X-ray diffraction method for their single crystals. The crystal structures are closely related to the scheelite type, with Ag+ and Ln3+ ions in statistical distribution.3 Shi et al. reported that the temperature dependence of the magnetic susceptibilities for AgLnMo2O8
(Ln = Ce–Nd, Sm, Gd and Tb) reveals Curie–Weiss law behavior with three anomalies occurring at 100, 160 and 230 K, which are observed in all samples investigated in the temperature range studied (77 ≤ T ≤ 300 K).4,5

![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) could be prepared as single-phase materials. Fig. 2 shows the X-ray diffraction pattern for AgGdMo2O8, as an example. We have performed Rietveld analysis for the XRD data. Table 1 lists the lattice parameters for AgLnMo2O8. The lattice parameters for AgLnMo2O8 are in excellent agreement with those reported by other workers.2–5 It has been found that the silver and lanthanide atoms randomly occupy the 2b and 2d sites. The (1/2Ag + 1/2Ln)1, (1/2Ag + 1/2Ln)2,
Mo1, Mo2, O1 and O2 ions occupy the 2d(1/2,0,1/4), 2b(1/2,1/2,0), 2a(0,0,0), 2c(0,1/2,1/4), 8g(x,y,z), and 8g(x,y,z) sites, respectively. The crystallographic positional parameters for AgLnMo2O8 after refinement are listed in Table 1.
could be prepared as single-phase materials. Fig. 2 shows the X-ray diffraction pattern for AgGdMo2O8, as an example. We have performed Rietveld analysis for the XRD data. Table 1 lists the lattice parameters for AgLnMo2O8. The lattice parameters for AgLnMo2O8 are in excellent agreement with those reported by other workers.2–5 It has been found that the silver and lanthanide atoms randomly occupy the 2b and 2d sites. The (1/2Ag + 1/2Ln)1, (1/2Ag + 1/2Ln)2,
Mo1, Mo2, O1 and O2 ions occupy the 2d(1/2,0,1/4), 2b(1/2,1/2,0), 2a(0,0,0), 2c(0,1/2,1/4), 8g(x,y,z), and 8g(x,y,z) sites, respectively. The crystallographic positional parameters for AgLnMo2O8 after refinement are listed in Table 1.







![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) symmetry), therefore, the electric field gradient (EFG) tensor at this site is axially symmetric (asymmetry parameter, η = 0). The hyperfine quadrupole interaction for this axial EFG can be written as shown in eqn. (3):
symmetry), therefore, the electric field gradient (EFG) tensor at this site is axially symmetric (asymmetry parameter, η = 0). The hyperfine quadrupole interaction for this axial EFG can be written as shown in eqn. (3):




