Modified direct injection high efficiency nebulizer with minimized dead volume for the analysis of biological samples by micro- and nano-LC-ICP-MS
Mathias
Wind
a,
Andreas
Eisenmenger
b and
Wolf D.
Lehmann
*a
aCentral Spectroscopy, German Cancer Research Centre (DKFZ), Im Neuenheimer Feld 280, D-69120, Heidelberg, Germany. E-mail: wolf.lehmann@dkfz.de
bDepartment of Biophysics and Medical Radiation Physics, German Cancer Research Centre (DKFZ), Im Neuenheimer Feld 280, 69120, Heidelberg, Germany
First published on 6th December 2001
Abstract
A direct injection high efficiency nebulizer (DIHEN) was modified for µLC-ICP-MS by inserting an additional internal capillary with od 90 µm and id 20 µm to minimize the interface dead volume. The modified DIHEN was compared with a conventional microflow nebulizer with a spray chamber interface with respect to sensitivity, signal stability and organic modifier dependency at flow rates ranging from 0.5 to 5 µl min−1. Although the modified DIHEN was slightly less sensitive, its signal stability was superior, its modifier dependency was less pronounced and its chromatographic resolution was much better due to its extremely small dead volume. These characteristics were demonstrated by the analysis of a mixture of synthetic phosphopeptides (31P detection) and synthetic thyroxine (127I detection). The influence of an organic modifier in the analyte solvent on the detection sensitivity was tested using the bioelements sulfur, phosphorus and iodine as examples. Phosphorus and iodine show an increased signal proportional to the amount of organic modifier with an intensity maximum of between 40% and 60% of modifier, which corresponds to a metal-like characteristic. For the detection of sulfur this characteristic is counteracted by a signal suppression effect proportional to the amount of organic modifier, which is caused by the formation of polyatomic species such as sulfur oxide.
Introduction
Over the past ten years LC-ICP-MS has become a powerful tool in chemical and biochemical analysis.1,2 The method offers both a species- and an element-specific analytical result. In addition to qualitative information, LC-ICP-MS provides the option for a direct quantification.In particular, qualitative and quantitative species analysis of organometallic substances containing selenium,3,4 arsenic,5,6 antimony7 and non-metals8,9 have been performed successfully using reversed phase and ion exchange chromatography coupled to ICP-MS.
Additionally, several separation techniques have been coupled to ICP-MS for biochemical analysis, e.g., HPLC or HPCE (high-performance capillary electrophoresis) for the analysis of cobalamines.10,11,12 Pharmaceutical analyses can also be performed in an effective and sensitive way using LC-ICP-MS.13,14
Chromatographic separation of complex biological samples combined with element detection results is a type of elemental profiling. For example, the element distribution in blood serum15,16 or the distribution of Cl, Br and I in humic acid17 have been established in this way. So far only a few studies have addressed the issue of sample stoichiometry. Among these are the analysis of metal cofactors in enzymes,18 the quantification of DNA adducts19 by 31P detection or determination of the protein phosphorylation20,21 by detection by 31P and 32S.
The analysis of biological samples is a particular challenge in that the matrix is usually highly complex and the analyte is present in low amounts only. The limited sample amount allows only a limited number of analytical runs to be made in which the analyte has to be both identified and quantified. This task often requires the use of organic and inorganic mass spectrometry methods coupled to HPLC7,20–23 and this approach is most powerful when a single sensitive LC method can be coupled alternately to ICP-MS and electrospray ionization mass spectrometry (ESI-MS). However, LC-ICP-MS combinations usually require flow rates in excess of 50 µl min−1 whereas LC-ESI-MS shows its optimal sensitivity at flow rates below 1 µl min−1. We believe that capillary LC with a flow rate of 4 µl min−1 represents a compromise, providing excellent results for both LC-ICP-MS and LC-ESI-MS combinations, as demonstrated recently.20,21
In addition, the choice of capillary LC shows several other beneficial aspects. Using ICP-MS the complete LC eluent can be introduced without oxygenation into the nebulizer, even in gradient reversed phase chromatography. A capillary LC system optimized for a flow rate of 4 µl min−1 shows greatly reduced adsorption and dilution effects and better sensitivity compared with conventional HPLC systems and thus this setup is much better suited for the analysis of biological samples.
Sample introduction into ICP-MS with flow rates of several µl min−1 has been performed either using interfaces initially constructed for much higher flow rates, or these studies were performed using in-house constructed devices that are not commercially available.24–26
The present work focuses on the properties of two different types of nebulizers operated at flow rates typical for capillary LC. The nebulizer types selected are a standard microflow nebulizer equipped with a spray chamber and a direct injection high efficiency nebulizer (DIHEN)27,28 modified by insertion of an additional internal capillary. Both interfaces were characterized with respect to the sensitivity, the signal stability and the gradient dynamics. As examples of applications the characterization of a synthetic phosphopeptide mixture and quality control of a thyroid gland hormone14 are demonstrated.
Experimental
ICP-MS system
All measurements were performed on a high resolution sector field mass spectrometer type Element2 (Thermo Finnnigan MAT GmbH, Bremen, Germany) at a medium resolution of 4000, which is sufficient for interference-free detection of phosphorus and iodine.Capillary HPLC system and direct introduction system
In this study the two nebulizers were tested with two different solvent delivery systems: a gradient HPLC pump connected with a capillary column and a syringe pump for direct introduction of a solvent.For HPLC a dual-syringe solvent delivery system (type 140B, Applied Biosystems, Foster City, CA, USA) was used. Samples were injected using a 5 µl stainless steel sample loop. For separation of the phosphopeptide mixture, a Vydac C18 column (0.3 mm × 250 mm, 5 µm, 300 Å, Grom, Herrenberg, Germany) was used. Thyroxine determination was performed on a Vydac C18 column (0.3 mm × 125 mm, 5 µm, 300 Å, LC Packings, Amsterdam, The Netherlands). The standard gradient used was 0–5 min, 5% B isocratic; 5–50 min, 5 to 100% B linear. Mobile phase A was water–trifluoroacetic acid 100 + 0.065 (v/v) and B was acetonitrile–water–trifluoroacetic acid 80 + 20 + 0.05 (v/v/v). Solvents were degassed by helium. The total flow was 116 µl min−1 and 80 µl min−1, using split ratios of about 1∶30 and 1∶20 to achieve a flow of about 4 µl min−1 over the 250 mm and the 125 mm columns, respectively. To regulate the flow rate the pre-split flow of the solvent delivery system was varied.
For direct introduction experiments a syringe pump type Harvard 01760 equipped with a 250 µl syringe (both from Harvard Apparatus, Holliston, MA, USA) was employed. As the connection a 280 µm od and 100 µm id fused silica capillary was used.
µLC-ICP-MS with spray chamber nebulizer
A self aspirating spray chamber nebulizer (Microflow PFA-LC Nebulizer with a zero dead volume connector for LC-ICP-MS 1006) in combination with a low-volume spray chamber (PFA Spray Chamber for Microflow, both from Elemental Scientific, Omaha, NE, USA) was used as the reference nebulization system. This coupled the µLC system via the integrated 1/8″ fitting to the ICP-MS. The parameters were optimized on the 238U signal by introducing the tuning solution mentioned below. The solution was introduced at a flow rate of 5 µl min−1. Average optimized tuning parameters are shown in Table 1.| Parameter | Spray chamber nebulizer | Modified DIHEN |
|---|---|---|
| Cool gas/l min−1 | 15.5 | 15.5 |
| Auxiliary gas/l min−1 | 1.16 | 0.90 |
| Sample gas/l min−1 | 1.155 | 0.360 |
| Plasma power/W | 1117 | 1050 |
| Resolution m/Δm | 4000 | 4000 |
| Samples per peak | 10 | 10 |
| Sample time/s | 0.04 | 0.04 |
| Detection frequency/s−1 | 0.5 | 0.5 |
µLC-ICP-MS with modified DIHEN
To couple a micro- or a nano-LC system to ICP-MS, the conventional DIHEN (Quartz-DIHEN-170-AA, J. E. Meinhard Associates Inc., Santa Ana, CA, USA)27 is not usable because of the high dead volume in the glass body. Therefore, a narrow fused silica capillary, od 90 µm id 20 µm of about 25 cm length, was inserted into the glass capillary of the DIHEN. This additional capillary was coupled directly to the fused silica capillary outlet of the separation column by a low dead volume union. The configuration is shown in Fig. 1.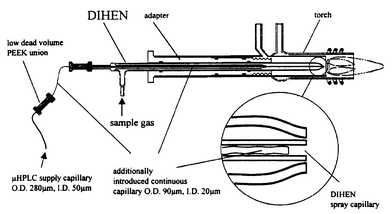 | ||
| Fig. 1 Scheme of the modified DIHEN nebulizer with one extra piece of capillary tubing (id 20 µm, od 90 µm) inserted into the integrated spray capillary of the DIHEN. The extra capillary is connected directly to the outlet of the LC column. | ||
Flow rates higher than 5 µl min−1 could not be established with the direct syringe introduction system because of the high back pressure of the 20 µm id capillary. The split ratio of the LC system had to be calibrated with the capillary in-line. Optimized parameters are summarized in Table 1.
A similar nebulizer for HPCE has been demonstrated for CE-ICP-MS.26
ESI-MS
Electrospray mass spectrometry was performed on a Q-TOF 2 instrument (Micromass, Manchester, UK) which was equipped with a nanoflow device. The spray voltage was about +1000 V. Spray capillaries for nano-ESI were prepared in-house using a micropipette puller type P-87 (Sutter Instruments, Novato, CA, USA) and coated with a semi-transparent film of gold in a sputter unit.29 Samples were dissolved in methanol–water–formic acid, 0.5 + 0.5 + 0.01.Chemicals and reagents
Water and acetonitrile were of HPLC grade from Merck (Darmstadt, Germany). Trifluoroacetic acid and all other chemicals were of analytical-reagent grade quality. Synthesis of phosphopeptides was performed by the F-moc technology in an automated peptide synthesizer AMS 422 (Abimed Analysentechnik, Langerfeld, Germany). The element mixture for metal measurements was the standard multi-element mixture for mass calibration (Merck). The signals of 115In (7.8 nM) and of 238U (4 nM) were used for the studies displayed in Fig. 2 and 3. Bis(4-nitro-phenyl)phosphate (BNPP) was from Aldrich (Milwaukee, WI, USA). Synthetic thyroxine was kindly supplied by F. Zehren (Peptido GmbH, Bexbach, Germany). For non-metal measurements, mixtures containing different ratios of LC eluents A and B including 10 µM caesium iodide, 10 µM NaH2PO4 and 10 µM cysteine were prepared. All other substances were from Merck. | ||
| Fig. 2 The normalized sensitivity obtained with (a) the modified DIHEN and (b) the spray chamber nebulizer at different sample flow rates of the aqueous tuning solution containing 7.8 nM 115In and 4 nM 238U. | ||
 | ||
| Fig. 3 Relative standard deviation of the signal intensity for (a) the modified DIHEN and (b) the spray chamber nebulizer at different sample flow rates of the aqueous tuning solution containing 7.8 nM 115In and 4 nM 238U. | ||
Results
Normalized sensitivity at different flow rates
The intensities of elements with low backgrounds were measured at several introduction flow rates to characterize the efficiency of the nebulization process. The results are displayed as the signal intensity normalized both to introduction flow rate and to concentration (unit: counts s−1 min µl−1 ppb−1), which is denoted as normalized sensitivity. For these sensitivity studies 115In and 238U were used since typical bioelements such as 31P or 32S exhibit high background intensities. The inorganic multi-element mixture (see Chemicals and reagents) was introduced at flow rates of between 0.5 and 5 µl min−1 to cover the typical flow rates of nano- and micro-LC. The interdependence between flow rate and normalized sensitivity is shown in Fig. 2 for the modified DIHEN and for the standard spray chamber nebulizer.Both systems were tuned to the maximum 238U intensity. The tuning parameters are given in Table 1. For both nebulizers the normalized sensitivity increases at higher flow rates. At 5 µl min−1 the standard nebulizer delivers a 238U normalized sensitivity of 450000 counts s−1 min µl−1 ppb−1, which is about twice as high as the value of the modified DIHEN with 225000 counts s−1 min µl−1 ppb−1.
At flow rates down to 0.5 µl min−1 the normalized sensitivity for the modified DIHEN decreases only by a factor of two whereas the value for the spray chamber nebulizer decreases by factor of 5 down to 1 µl min−1. Reproducible results could not be achieved below 1 µl min−1 using the spray chamber nebulizer due to strong signal pulsing. Repeated measurements showed reproducible results for both 238U and 115In.
With LC analysis the signal stability (standard deviation of the signal intensity) is the important parameter characterizing the limit of detection. By convention, a signal-to-noise ratio of three to one equals the limit of detection. For all measurements described above the relative standard deviation of the signals was calculated and the results are summarized in Fig. 3.
The relative standard deviation of the signal intensity increases with decreasing flow rate at flow rates lower than 2.5 µl min−1 for both nebulizers. At flow rates higher than 2.5 µl min−1 the relative standard deviation remains at a constant value. Over the complete range of flow rates studied the relative standard deviation of the modified DIHEN signal was about a factor of five smaller than that of the spray chamber nebulizer. This is probably due to the extremely narrow additional capillary of the modified DIHEN compared with the standard nebulizer, resulting in a more continuous spray process.
Influence of solvent composition, investigated by direct introduction experiments
The solvent composition has a significant effect on the signal intensity in ICP-MS.19,30 The signal intensity varies with changing organic modifier, especially when using a chromatographic gradient of water and acetonitrile. Moreover, the influence of solvent composition on signal intensity is different for each element because of different plasma conditions. To obtain representative data, the experiments were carried out with the main isotopes of three bioelements of general interest: 31P, 32S and 127I.Solutions consisting of the HPLC solvents A and B described above and containing the elements of interest at concentrations of 10 µM were introduced with a flow rate of 4 µl min−1. The experiments were performed in a range of between 0 and 90% of solvent B. Direct introduction of a set of solutions with a constant composition (stepwise variation) was preferred to a continuous variation as is obtained in gradient LC, in order to obtain an optimally defined experimental system. The results are shown in Fig. 4.
 | ||
| Fig. 4 Signal intensities of 127I, 31P, 32S, 32S16O for (a) the modified DIHEN and (b) the spray chamber nebulizer as a function of the solvent composition (solvent A: water, 0.065% trifluoroacetic acid; solvent B: 20% water, 80% acetonitrile, 0.05% trifluoroacetic acid) containing 10 µM iodide, 10 µM phosphate and 10 µM cysteine. | ||
The intensity is highly dependent on the matrix composition for both nebulizers. An intensity maximum occurs at 30% B for the DIHEN and at 60% B for the spray chamber nebulizer. This matrix dependency is shown to be less for the DIHEN. The gain in intensity between pure solvent A and the maximum intensity is about 1.5 for the DIHEN and more than 2.7 for the spray chamber nebulizer. Again, the modified DIHEN shows better stability. Iodine and phosphorus show the same characteristics whereas sulfur has a different behaviour, resulting in reduced intensity with increasing amounts of organic modifier.8,9 Therefore we checked for the presence of sulfur-containing polyatomic ions. Among these SO was observed as the most abundant species. For example, the 32S16O ion is not detectable in pure water but increases in intensity with increasing solvent B up to a maximum intensity for both nebulizers. The relative intensity of 32S16O to 32S increases over the entire gradient. Further polyatomic ions containing sulfur and oxygen are supposed to be formed. Again the relative standard deviation of the signal intensity for the modified DIHEN is about half the size as compared with the spray chamber nebulizer.
Limit of detection in µLC-ICP-MS for 31P detection
The limit of detection was determined by use of BNPP with µLC-ICP-MS for 31P. The reason for its use is the importance of phosphorus in biological samples, e.g., in nucleotides and phosphoproteins. Limits of detection were measured at an LC flow rate of 4 µl min−1, the standard flow of a conventional 300 µm µLC column. LC conditions are given under Experimental. BNPP elutes in the gradient used at 32 min with the spray chamber nebulizer and at 34 min with the modified DIHEN nebulizer. Aqueous solutions of 0.5 and 1 pmol BNPP were injected and the resulting signal-to-noise ratios were determined. Using these data the limit of detection for a signal-to-noise ratio of 3 was calculated. The limits of detection were 40 fmol and 55 fmol for the spray chamber nebulizer and the modified DIHEN, respectively. In addition to the introduction experiments, the LC-ICP-MS experiment also showed similar limits of detection for both nebulizers although the relative standard deviation was better for the modified DIHEN.µLC-ICP-MS of a phosphopeptide mixture
A mixture of several synthetic phosphorylated peptides was analyzed by µLC-ICP-MS using a gradient separation and 31P detection to test the influence of the nebulizers on the chromatographic resolution. The phosphopeptide sequences are listed in Table 2.| Compound number | Substance |
|---|---|
| 1 | SPRS-Sp-ATEEN |
| 2 | SPE-Sp-SDTEEN |
| 3 | QAAAA-Sp-AAAAQ |
| 4 | GNAAAAKKG-Sp-EQESVK |
| 5 | EAQAA-Yp-AAQAK |
| 6 | PTPSAP-Sp-PQPK |
| 7 | impurity of 8 |
| 8 | LRRA-Tp-LG |
| 9 | KG-Sp-EQESVKEFLAK |
| 10 | FKGPGDTSNFDDDDIRV-Sp-INEK |
| 11 | TW-Tp-LCGTPEY |
The chromatograms observed after injection of 5–20 pmol of each peptide and using a flow rate of 4 µl min−1 are given in Fig. 5(a) for the modified DIHEN and in Fig. 5(b) for the spray chamber nebulizer.
 | ||
| Fig. 5 Separation of a mixture of synthetic phosphopeptides (see Table 2) by µLC-ICP-MS and 31P detection for (a) the modified DIHEN and (b) the spray chamber nebulizer. | ||
The intensities of the tops of the peaks are similar for both nebulizers, but the chromatographic resolution is much better for the modified DIHEN, as is evident in particular for substance pairs 1, 2 and 7, 8 which are not separated using the spray chamber nebulizer. The baseline stability is excellent for the chromatogram using the modified DIHEN. An increase in the baseline noise over the gradient could not be noticed for the modified DIHEN, whereas the spray chamber nebulizer shows a much higher baseline noise at the end of the gradient. The retention times are about 1–2 min higher using the modified DIHEN, due to adsorption effects in the additionally introduced 20 µm id capillary.
Determination of synthetic thyroxine
As a further application, synthetic thyroxine (T4), a hormone from the thyroid gland, was measured by µLC-ICP-MS with 127I detection14 and nano-ESI-MS to demonstrate the capability of this method for trace analysis. Thyroxine (T4) and triiodothyronine (T3) contain 4 and 3 atoms of iodine per molecule, respectively. The limit of detection was calculated to be about 40 fmol of injected iodine. This corresponds to a limit of detection of about 10 fmol for thyroxine. Fig. 6(a) shows the 127I trace of the µLC-ICP-MS run after injection of 5 pmol of thyroxine which elutes at 31 min. Expanding the y-axis by a factor of 85, a smaller peak at 28.5 min becomes visible, as shown in Fig. 6(b). This peak represents triiodothyronine (T3) according to the elution order in reversed phase chromatography.14 The relative ratio of T3 to T4 is calculated to be about 0.25%, representing about 12 fmol of triiodothyronine injected. | ||
| Fig. 6 Quality control of thyroxine by µLC-ICP-MS and nano-ESI-MS: (a) 127I trace of about 5 pmol T4 (thyroxine) by µLC-ICP-MS using the modified DIHEN; (b) expansion by a factor of 85 of trace (a) showing the T3 (triiodothyronine); (c) nano-ESI mass spectrum of T4; (d) expansion by a factor of 85 of spectrum (c) showing the T3 (triiodothyronine) signal. For peak information see Table 3. | ||
To confirm this result nano-ESI-MS was also used to determine the T3 to T4 ratio. A 10 µM thyroxine sample, a concentration ten times higher than that used for the LC-ICP-MS experiment, was determined by nano-ESI-MS and the spectrum observed is displayed in Fig. 6(c). The base peak at m/z 777.8 represents the singly charged thyroxine ion [M + H]+. In addition to this, the sodium adduct [M + Na]+ of thyroxine, the disodium adduct [M–H + 2Na]+, the thyroxine immonium ion [M + H–CO2H2]+, and the ion [M + H–NH3]+ can be observed. These ions are formed by the ionization process and are not impurities present in the sample. Detailed information is given in Table 3.
On expanding the y-axis of the nano-ESI-MS spectrum in Fig. 6(c) by a factor of 85, a signal for triiodothyronine can be found at m/z 651.99. Considering all relevant peaks the ratio of T3 to T4 was estimated to be 0.24%, which is in good agreement with the µLC-ICP-MS result. Peak identity was confirmed by additional nano-ESI tandem mass spectrometry.
Conclusion
The modified DIHEN is less dependent on introduction rate and solvent composition compared with the spray chamber nebulizer. The modified DIHEN nebulizer offers the possibility of direct LC-ICP-MS measurements in the micro- and nano-LC mode without the use of any make up flow, and thus is well suited to the analysis of biological samples. In general the special construction with minimized dead volume retains the chromatographic resolution even at microliter and submicroliter flow rates, avoiding the deteriorating extra column effect of spray chamber nebulizers.Acknowledgements
We are indebted to H. P. Beck for encouraging support and to R. Pipkorn for the synthetic phosphopeptides.References
- K. Sutton, R. M. Sutton and J. A. Caruso, J. Chromatogr. A, 1997, 789, 85–126 CrossRef CAS.
- K. L. Sutton and J. A. Caruso, J. Chromatogr. A, 1999, 856, 243–258 CrossRef CAS.
- B. Mischalke, Fresenius' J. Anal. Chem., 1995, 351, 670–677 CrossRef CAS.
- J. M. Marchante-Gayón, C. Thomas, I. Feldmann and N. Jakubowski, J. Anal. At. Spectrom., 2000, 15, 1093–1102 RSC.
- S. Wankran and S. A. Pergantis, J. Anal. At. Spectrom., 2000, 15, 625–633 Search PubMed.
- K. Falk and H. Emons, J. Anal. At. Spectrom., 2000, 15, 643–649 RSC.
- J. Lintschinger, O. Schramel and A. Kettrup, Fresenius' J. Anal. Chem., 1998, 361, 96–102 CrossRef CAS.
- B. Divjak and W. Goessler, J. Chromatogr. A, 1999, 844, 161–169 CrossRef CAS.
- S.-J. Jiang and R. S. Houk, Spectrochim. Acta, Part B, 1988, 43, 405–411 CrossRef.
- S. A. Baker and N. J. Miller-Ihli, Spectrochim. Acta Part B, 2000, 55, 1823–1832 CrossRef.
- H. Chassaigne and J. Szpunar, Analusis, 1998, 26, M48–M51 Search PubMed.
- A. Tangen and W. Lund, J. Chromatogr. A, 2000, 891, 129–138 CrossRef CAS.
- B.-O. Axelsson, M. Jörnten-Karlsson, P. Michelsen and F. Abou-Shakra, Rapid Commun. Mass Spectrom., 2001, 15, 375–385 CrossRef CAS.
- K. Takatera and T. Wantanabe, Anal. Chem., 1993, 65, 759–762 CrossRef CAS.
- K. Inagaki, T. Umemura, H. Matsuura and H. Haraguchi, Anal. Sci., 2000, 16, 787–788 Search PubMed.
- M. M. Bayón, A. B. S. Cabezuelo, E. B. González, J. I. G. Alonso and A. Sanz-Medel, J. Anal. At. Spectrom., 1999, 14, 947–951 RSC.
- G. Rädlinger and K. G. Heumann, Fresenius' J. Anal. Chem., 1997, 395, 430–433 CrossRef CAS.
- A. Leber, B. Hemmens, B. Klosch, W. Goessler, G. Raber, B. Mayer and K. Schmidt, J. Biol. Chem., 1999, 53, 37658–37664 CrossRef CAS.
- C. Siethoff, I. Feldmann, N. Jakubowski and M. Linscheid, J. Mass Spectrom., 1999, 43, 421–426 CrossRef.
- M. Wind, M. Edler, N. Jakubowski, M. Linscheid, H. Wesch and W. D. Lehmann, Anal. Chem., 2001, 73, 29–35 CrossRef CAS.
- M. Wind, H. Wesch and W. D. Lehmann, Anal. Chem., 2001, 73, 3006–3010 CrossRef CAS.
- H. Chassaigne and R. Lobinski, Fresenius' J. Anal. Chem., 1998, 361, 267–273 CrossRef CAS.
- V. Vaccina, R. Lobinski, M. Oven and M. H. Zenk, J. Anal. At. Spectrom., 2000, 15, 529–534 RSC.
- L. Wang, S. W. May and R. F. Browner, J. Anal. At. Spectrom., 1996, 11, 1137–1146 RSC.
- M. A. Tarr, G. Zhu and R. F. Browner, Anal. Chem., 1993, 65, 1689–1695 CrossRef CAS.
- L. Bendahl, B. Gammelgaard, O. Jøns, O. Farver and S. H. Hansen, J. Anal. At. Spectrom., 2001, 16, 38–42 RSC.
- J. A. McLean, H. Zhang and A. Montaser, Anal. Chem., 1998, 70, 1012–1020 CrossRef CAS.
- B. W. Acon, J. A. McLean and A. Montaser, Anal. Chem., 2000, 72, 1885–1893 CrossRef CAS.
- M. Wilm and M. Mann, Anal. Chem., 1996, 68, 1–8 CrossRef CAS.
- K. L. Ackley, K. L. Sutton and J. A. Caruso, J. Anal. At. Spectrom., 2000, 15, 1069–1073 RSC.
| This journal is © The Royal Society of Chemistry 2002 |
