Efficient solvent-free Thorpe reactions
Kazuhiro Yoshizawa, Shinji Toyota and Fumio Toda*
Department of Chemistry, Faculty of Science, Okayama University of Science, Ridai-cho 1-1, Okayama, 700-0005, Japan. E-mail: toda@chem.ous.ac.jp
First published on 8th February 2002
Abstract
Solvent-free intermolecular and intramolecular Thorpe reactions proceeded efficiently to give acyclic and cyclic enamines, respectively. In the latter case, the reaction products were obtained as colorless crystalline powders just by washing of the reaction mixture with water.
Green ContextThe avoidance of volatile organic solvents is one of the major targets of green chemistry. While there is considerable and worthwhile research effort going into the design and application of alternative solvents it is worth remembering that the fundamentals of green chemistry teach us to seek to avoid auxiliaries, including solvents, in chemical manufacturing processes. Here we see a good example of how some important organic reactions can be efficiently accomplished without any solvent. Various solvent free reactions of nitriles are described. The reaction procedure is simple and the product yields are good. There is however still room for improvement since quite large amounts of conventional strong base are required and in some cases, an organic solvent is used to extract the product at the end of the reaction.JHC |
Introduction
A solvent-free organic reaction is an important synthetic procedure from the view point of green and sustainable chemistry. We have been developing various solvent-free organic reactions.1 Recently, we found that the intermolecular dimerization of nitriles and intramolecular cyclization of dinitriles, which are known as Thorpe reactions,2 proceed very efficiently under solvent-free conditions. When the reaction product is a solid, it can be isolated just by washing the reaction mixture with water. The solvent-free procedure in Thorpe reactions is valuable not only for ecological and economical reasons but also for simplicity in procedure and for the high yields of the products. In addition, the reaction mechanism of the solvent-free Thorpe reaction was clarified by monitoring of the reaction by IR spectral measurement in the solid state.Results and discussion
Intermolecular Thorpe reactions
For example, after a mixture of acetonitrile (1) and a 1.4 molar amounts of powdered tBuOK was kept at room temperature overnight, water was added to the reaction mixture and the product extracted with ether. Distillation using Kugelrohr apparatus of the residue left after evaporation of the solvent gave a 1∶1 mixture of (E)- and (Z)-enamines (2) in 48% yield (Scheme 1). The same treatment of propionitrile (3) with powdered tBuOK gave a 1∶1 mixture of (E)- and (Z)-enamines (4) in 65% yield. Solvent-free Thorpe reaction of benzylcyanide (5) at 80 °C was completed within 3 h to give a 4∶1 mixture of (E)- and (Z)-enamines (6) in 73% yield. For both 2 and 4, the ratio of (E)- and (Z)-isomers was determined to be 1∶1 by 1H NMR spectroscopy, although the correlation between the spectral data and the isomerism is not clear. In the case of 6, however, the (E)∶(Z) ratio was determined to be 4∶1 by 1H NMR spectroscopy and the spectrum which shows normal CH2 absorption was assigned to the (E)-6 isomer. The (Z)-6 isomer showed CH2 protons at relatively higher magnetic fields due to a shielding effect by the phenyl ring. The formation of the sterically more favorable (E)-6 isomer as a major product is resonable. In the Thorpe reactions of 1, 3 and 5 reported in 1942, products had been identified as imines instead of enamines and no (E), (Z) assignment had been described.3 In 1959, the enamine structure 2 was assigned to the Thorpe reaction product .4 | ||
| Scheme 1 | ||
The solvent-free procedure can be applied to cross-Thorpe reactions (Scheme 2). A mixture of powdered p-methylbenzonitrile (7b), two molar amounts of 1 and two molar amounts of powdered tBuOK was heated at 80 °C for 2 h and kept at room temperature overnight. To the reaction mixture, water was added and the product extracted with ether. From the ether solution, 8b was obtained by distillation in 74% yield. In this reaction, the product was obtained only as the (E)-isomer. In the solvent-free cross-reaction, self-condensation reaction of 1 did not occur. On the other hand, the cross-reaction of 7b and 1 under reflux in toluene and in THF for 1 day gave 8b in 52 and 34% yields, respectively.
 | ||
| Scheme 2 | ||
The cross-condensation of benzonitrile (7a) with 1 under solvent-free conditions gave 8a in 70% yield. The same cross-condensation of 7a and 7b with 5 gave 9a and 9b in 70 and 62% yields, respectively. In all reactions, no (Z)-isomer was produced.
Intramolecular Thorpe reactions
After a mixture of powdered adiponitrile (10) and 0.60 molar amount of powdered tBuOK was kept at room temperature for 3 h, the reaction mixture was washed with water to give the cyclization product (11) as a white crystalline powder in 74% yield (Scheme 3). It has been reported that the Thorpe reaction of 10 is very sensitive to the reaction conditions. For example, the reaction under homogeneous conditions using a catalytic amount of tBuONa in tBuOH gave mostly a dimeric product in 67–76% yield, however, the reaction under heterogeneous conditions using an equimolar amount of tBuONa in toluene gave 11 in 85% yield.5 The heterogeneous reaction of 10 in toluene corresponds to the solvent-free reaction which gives 11 as the sole product. By the same solvent-free procedure, pimelonitrile (12) and o-di(cyanomethyl)benzene (14) gave 13 and 18 as white crystalline powders in 83 and 97% yields, respectively. It has been reported that the Thorpe reaction of 14 in EtOH containing EtONa gives 18 in a quantitative yield.6 | ||
| Scheme 3 | ||
Until recently the products of all nitrile cyclizations by the Thorpe reaction had been formulated as imines. In 1955, Hammer and Hines pointed out that the product from adiponitrile (10) was better described as the enamine (11). By the same idea, the cyclization product of 14 can be described as the enamine (18) which is derived from the initially formed imine (17) through the mechanism shown in Scheme 3. In order to clarify the mechanism, the intramolecular Thorpe reaction of 14 under solvent-free conditions was monitored by measurement of IR spectra in Nujol mulls (Fig. 1). As the reaction proceeds, the CN absorption of 14 at 2250 cm−1 decreases and a new CN absorption of the intermediate (17) arises at 2143 cm−1. As 17 is converted into 18 by a proton migration, the CN absorption of 17 at 2143 cm−1 disappears, and only the CN absorption of 18 at 2189 cm−1 remains.
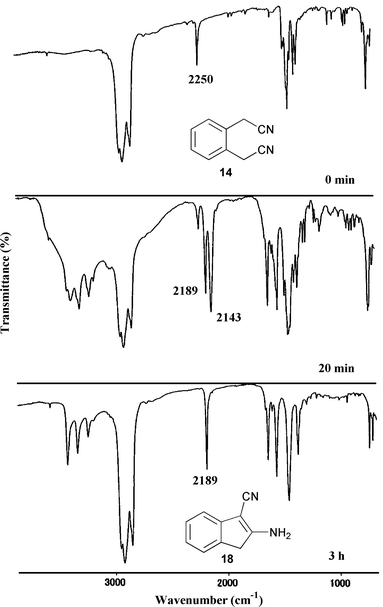 | ||
| Fig. 1 Monitoring of the Thorpe reaction of 14 to 18via17 by IR spectral measurement in Nujol mulls. | ||
Experimental
General procedures
All 1H NMR and 13C NMR spectra were measured in CDCl3.Intermolecular Thorpe reactions of acetonitrile (1), propionitrile (3) and benzylcyanide (5)
A mixture of nitrile (0.10 mol) and tBuOK (0.14 mol) was kept at room temperature overnight. In the case of 5, reaction was carried out at 80 °C for 2 h. Water was added to the reaction mixture and the product extracted with diethyl ether. Distillation using Kugelrohr apparatus of the residue left after evaporation of the solvent of the dried ether solution gave the enaminonitrile condensation product as an oil in the yield indicated in Table 1. The (E)∶(Z) ratios were determined by 1H NMR spectra.2 (1∶1 (E),(Z) mixture): 48% yield; IR (neat) 1600, 1647, 2181, 3253, 3353, 3419 cm−1. The IR spectrum was identical to that reported for 2.41H NMR (400 MHz) one isomer: δ 1.92 (s, 3 H, CH3), 3.82 (s, 1 H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.64 (br s, 2 H, NH2); the other isomer: δ 2.10 (s, 3 H, CH3), 4.12 (s, 1 H,
CH), 4.64 (br s, 2 H, NH2); the other isomer: δ 2.10 (s, 3 H, CH3), 4.12 (s, 1 H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.28 (br s, 2 H, NH2). 4 (1:1 (E),(Z) mixture): 65% yield; IR (neat) 1613, 1641, 2182, 3232, 3361, 3457 cm−1. The IR spectrum was identical to that reported for 4.71H NMR (300 MHz) one isomer: δ 1.19 (t, J
=7.6 Hz, 3 H, CH3), 1.69 (s, 3 H, CH3), 2.44 (q, J = 7.6 Hz, 2 H, CH2), 4.04 (br s, 2 H, NH2); the other isomer: 1.23 (t, J
= 7.6 Hz, 3 H, CH3), 1.74 (s, 3 H, CH3), 2.21 (q, J = 7.6 Hz, 2 H, CH2), 4.31 (br s, 2 H, NH2). 6 (4∶1 (E),(Z) mixture): 73% yield; IR (neat) 1583, 1626, 2180, 3234, 3346, 3467 cm−1. The IR spectrum was identical to that reported for 6.31H NMR (300 MHz) (E)-isomer: δ 3.96 (s, 2 H, CH2), 4.51 (br s, 2 H, NH2), 7.25–7.44 (m, 10 H, ArH); (Z)-isomer: δ 3.70 (s, 2 H, CH2), 4.69 (br s, 2 H, NH2), 7.25–7.44 (m, 10 H, ArH).
CH), 4.28 (br s, 2 H, NH2). 4 (1:1 (E),(Z) mixture): 65% yield; IR (neat) 1613, 1641, 2182, 3232, 3361, 3457 cm−1. The IR spectrum was identical to that reported for 4.71H NMR (300 MHz) one isomer: δ 1.19 (t, J
=7.6 Hz, 3 H, CH3), 1.69 (s, 3 H, CH3), 2.44 (q, J = 7.6 Hz, 2 H, CH2), 4.04 (br s, 2 H, NH2); the other isomer: 1.23 (t, J
= 7.6 Hz, 3 H, CH3), 1.74 (s, 3 H, CH3), 2.21 (q, J = 7.6 Hz, 2 H, CH2), 4.31 (br s, 2 H, NH2). 6 (4∶1 (E),(Z) mixture): 73% yield; IR (neat) 1583, 1626, 2180, 3234, 3346, 3467 cm−1. The IR spectrum was identical to that reported for 6.31H NMR (300 MHz) (E)-isomer: δ 3.96 (s, 2 H, CH2), 4.51 (br s, 2 H, NH2), 7.25–7.44 (m, 10 H, ArH); (Z)-isomer: δ 3.70 (s, 2 H, CH2), 4.69 (br s, 2 H, NH2), 7.25–7.44 (m, 10 H, ArH).
Cross-Thorpe reactions of benzonitrile (7a) and p-methylbenzonitrile (7b) with acetonitrile (1) and benzylcyanide (5)
A mixture of two different nitriles A and B (0.10 mol each) and powdered tBuOK (0.28 mol) was heated at 80 °C for 3 h and then kept at room temperature overnight. Water was added to the reaction mixture and the product extracted with ether. Hexane was added to the ether solution and kept at room temperature to give the cross-Thorpe reaction product as crystals in the yields indicated below.8a: 70% yield; mp 89–90 °C; IR (Nujol) 1588, 1628, 2180, 3351, 3442 cm−1; 1H NMR (400 MHz) δ 4.25 (s, 1 H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.94 (br s, 2 H, NH2), 7.41–7.51 (m, 5 H, ArH); 13C NMR (100 MHz) δ 63.8, 119.4, 126.0, 129.0, 130.9, 135.4, 161.4. 8b: 52% yield; mp 107–108 °C; IR (Nujol) 1588, 1641, 2182, 3243, 3341, 3430 cm−1; 1H NMR (400 MHz) δ 2.39 (s, 3 H, CH3), 4.24 (s, 1 H,
CH), 4.94 (br s, 2 H, NH2), 7.41–7.51 (m, 5 H, ArH); 13C NMR (100 MHz) δ 63.8, 119.4, 126.0, 129.0, 130.9, 135.4, 161.4. 8b: 52% yield; mp 107–108 °C; IR (Nujol) 1588, 1641, 2182, 3243, 3341, 3430 cm−1; 1H NMR (400 MHz) δ 2.39 (s, 3 H, CH3), 4.24 (s, 1 H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.89 (br s, 2 H, NH2), 7.43 (t, J = 8.2 Hz, 2 H, ArH), 7.39 (d, J = 8.2 Hz, 2 H, ArH); 13C NMR (100 MHz) δ 21.3, 63.0, 119.6, 125.8, 129.6, 132.4, 141.3, 161.5. 9a: 70% yield; mp 147–148 °C; IR (Nujol) 1556, 1619, 2176, 3201, 3303, 3345, 3467 cm−1; 1H NMR (400 MHz) δ 4.78 (br s, 2 H, NH2), 7.28–7.50 (m,
8 H, ArH), 7.69–7.72 (m, 2 H, ArH); 13C NMR (75 MHz) δ 81.1, 122.4, 127.2, 127.9, 128.4, 128.6, 129.2, 133.8, 135.8, 157.1. 9b: 62% yield; mp 135–136 °C; IR (Nujol) 1555, 1606, 2182, 3389, 3492 cm−1; 1H NMR (400 MHz) δ 2.41 (s, 3 H, CH3), 4.73 (br s, 2 H, NH2), 7.27–7.31 (m, 3 H, ArH), 7.43 (t, J = 7.9 Hz, 2 H, ArH), 7.54 (d, J = 8.0 Hz, 2 H, ArH), 7.60 (t, J = 8.1 Hz, 2 H, ArH); 13C NMR (100 MHz) δ 21.4, 81.6, 122.3, 127.3, 127.9, 128.6, 129.4, 129.5, 133.1, 134.1, 140.9, 157.0.
CH), 4.89 (br s, 2 H, NH2), 7.43 (t, J = 8.2 Hz, 2 H, ArH), 7.39 (d, J = 8.2 Hz, 2 H, ArH); 13C NMR (100 MHz) δ 21.3, 63.0, 119.6, 125.8, 129.6, 132.4, 141.3, 161.5. 9a: 70% yield; mp 147–148 °C; IR (Nujol) 1556, 1619, 2176, 3201, 3303, 3345, 3467 cm−1; 1H NMR (400 MHz) δ 4.78 (br s, 2 H, NH2), 7.28–7.50 (m,
8 H, ArH), 7.69–7.72 (m, 2 H, ArH); 13C NMR (75 MHz) δ 81.1, 122.4, 127.2, 127.9, 128.4, 128.6, 129.2, 133.8, 135.8, 157.1. 9b: 62% yield; mp 135–136 °C; IR (Nujol) 1555, 1606, 2182, 3389, 3492 cm−1; 1H NMR (400 MHz) δ 2.41 (s, 3 H, CH3), 4.73 (br s, 2 H, NH2), 7.27–7.31 (m, 3 H, ArH), 7.43 (t, J = 7.9 Hz, 2 H, ArH), 7.54 (d, J = 8.0 Hz, 2 H, ArH), 7.60 (t, J = 8.1 Hz, 2 H, ArH); 13C NMR (100 MHz) δ 21.4, 81.6, 122.3, 127.3, 127.9, 128.6, 129.4, 129.5, 133.1, 134.1, 140.9, 157.0.
Intramolecular Thorpe reactions of adiponitrile (10), pimelonitrile (12) and o-di(cyanomethyl)benzene (14)
A mixture of adiponitrile (10) (2.16 g, 20 mmol) and powdered tBuOK (3.16 g, 24 mmol) was kept at room temperature overnight. The crystalline solid formed by addition of water to the reaction mixture was filtered off and recrystallized from MeOH to give the cyclization product (11) (1.60 g) in 74% yield as a colorless crystalline powder: mp 147–148 °C (lit.,5 mp 147–148 °C); IR (Nujol) 1608, 1644, 2182, 3235, 3352, 3427 cm−1; 1H NMR (400 MHz) δ 1.89–1.96 (m, 2 H, CH2), 2.46 (t, J = 7.9 Hz, 2 H, CH2), 2.54 (t, J = 7.0 Hz, 2 H, CH2), 4.43 (br s, 2 H, NH2).A mixture of pimelonitrile (12) (1.22 g, 10 mmol) and powdered tBuOK (1.58 g, 12 mmol) was kept at 80 °C for 3 h and then at room temperature overnight. Water was added to the reaction mixture and the product extracted with ether. Recrystallization of the crystalline solid left after evaporation of the solvent of the ether solution from MeOH gave 13 as colorless crystals (1.01 g, 83% yield): mp 94 °C; IR (Nujol) 1604, 1643, 2172, 3225, 3346, 3436 cm−1; 1H NMR (300 MHz) δ 1.56–1.72 (m, 4 H, CH2), 2.10–2.21 (m, 4 H, CH2), 4.21 (br s, 2 H, NH2).
A mixture of o-di(cyanomethyl)benzene (14) (1.56 g, 10 mmol) and tBuOK (0.75 g, 6.0 mmol) was ground using an agate mortar and pestle for 5 min, and the mixture was kept at room temperature for 3 h. Washing the reaction mixture with water gave the cyclization product (18) (1.51 g, 97% yield): mp 192–193 °C (lit.,6 193 °C); IR (Nujol) 1567, 1645, 2189, 3244, 3339, 3426 cm−1; 1H NMR (300 MHz) δ 3.56 (s, 2 H, CH2), 5.12 (br s, 2 H, NH2), 7.03 (t, J = 7.3 Hz, 1 H, ArH), 7.16–7.28 (m, 3 H, ArH).
References
- F. Toda, Synlett, 1993, 302; F. Toda, Acc. Chem. Res., 1995, 28, 480 CrossRef CAS; F. Toda, Supramol. Sci., 1996, 3, 139 CrossRef CAS; F. Toda, Compr. Supramol. Chem., 1996, 6, 465 Search PubMed; K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025 CrossRef CAS.
- J. R. Scheffer and J. J. Bloomfield, Org. React., 1967, 15, 28 and references cited therein Search PubMed.
- H. Adkins and G. M. Whitman, J. Am. Chem. Soc., 1942, 64, 150 CrossRef CAS.
- P. Kurtg, H. Gold and H. Disselnkoetter, Liebigs Ann. Chem., 1959, 624, 1 Search PubMed.
- Q. E. Thompson, J. Am. Chem. Soc., 1958, 80, 5483 CrossRef CAS.
- C. W. Moore and J. F. Thorpe, J. Chem. Soc., 1908, 93, 165 Search PubMed.
- S. Boldwin, J. Org. Chem., 1961, 26, 3288 CrossRef.
| This journal is © The Royal Society of Chemistry 2002 |
