Bimetallic tris-oxalato-salts (n-CnH2n+1)PPh3MIIFeIII(C2O4)3 (n
= 3–7, MII
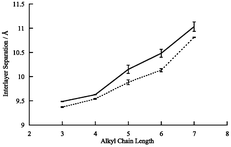
= Mn, Fe) were prepared and the structures investigated by powder X-ray diffraction in order to study the evolution of the structure and magnetic properties as a function of alkyl chain length. The compounds all have the same two-dimensional honeycomb structure of MII and FeIII bridged by oxalate, with the organic cations lying between the metal–oxalate layers, whose separation ranges from 9.48 Å (n
= 3) to 11.10 Å (n
= 7) for the FeII salts and 9.37 to 10.81 Å for MnII. The compounds all behave as ferrimagnets, with magnetic parameters similar to the corresponding AMIIFeIII(C2O4)3 with A =
NR4+, PPh4+ and Tcs almost insensitive to interlayer separation. The MnII salts exhibit uncompensated magnetisation below Tc and the FeII ones show Néel type N ferrimagnetism, with negative magnetisation at low temperature, the magnitude of which is influenced by the preparation conditions, due to vacancies in the FeII sublattice.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...