Structural transition in liquid dimethylsulfoxide induced by a high electrical field
S.
Markarian
*a,
Kh.
Nerkararyan
b and
W.
Fawcett
c
aDepartment of Chemistry and Yerevan State University, Al. Manoukian 1, 375049 Yerevan, Armenia. E-mail: fizkol@ysu.am
bDepartment of Physics, Yerevan State University, Al. Manoukian 1, 375049 Yerevan, Armenia
cDepartment of Chemistry and University of California, Davis, CA 95616, USA
First published on 13th December 2001
Abstract
A sharp change of the power of the output laser radiation during its propagation through a crystal–gap–crystal structure in the presence of a high applied electric field has been observed for liquid DMSO. The formation of large molecular aggregates on the surface of charged crystals is suggested to be responsible for this phenomenon.
Introduction
Dimethylsulfoxide (DMSO) has unique peculiarities and is of great interest from both scientific and practical viewpoints. DMSO and its aqueous solutions are widely used in the chemical industry, in biology and medicine, and in the field of environmental protection. One of the most important properties of DMSO as well as its nearest homologues (diethyl-, dipropyl-, dibutylsulfoxides) is their self-associative structure which exists both in the solid and liquid states, as well as in aqueous and non-aqueous solutions.1–10 It is this fact that brings about the anomalous physicochemical properties and peculiar reactivity of dialkylsulfoxides (DASO). Nevertheless the mechanism of association remains debatable for the time being. The existence of both cyclic dimers with an antiparallel orientation of the two dipole vectors and linear chains of associated DASO molecules due to intermolecular hydrogen bonding of the type CH⋯O![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) S has been discussed.7–9 On the basis of IR and Raman studies, Fini and Mirone10 suggested that liquid DMSO is composed of clusters inside of which the molecules are oriented in a partially ordered way.
S has been discussed.7–9 On the basis of IR and Raman studies, Fini and Mirone10 suggested that liquid DMSO is composed of clusters inside of which the molecules are oriented in a partially ordered way.
Obviously, study of the effect of external factors on the physicochemical and spectroscopic characteristics of DASO allows elucidation of the liquid structure of DASO. Recently, on the basis of Raman spectroscopic measurements, Czeslik and Jonas8 found that a pressure-induced increase occurs in the concentration of clusters with strongly associated molecules. They concluded that pressure favors the formation of aggregates in the liquid phase of DMSO which results in a more ordered local structure compared to the structure at ambient pressure. Moreover, DMSO has an ability of ordering above metal11,12 and silicia13 surfaces. From surface-enhanced Raman scattering and differential capacity measurements Shen and Pemberton11 inferred that DMSO formed an ordering head-to-tail array on the Ag surface. With the use of in situ scanning tunneling microscopy Si and Gewirth12 have shown that DMSO can form an ordered layer near the Au surface.
Early studies of the temperature dependence of 1H NMR spectra and light scattering measurements have shown that cluster formation of sulfoxide molecules takes place at low temperatures in solution.1
Experimental
The crystal–gap–crystal structure (CGCS) was created using a crystal of GaAs. The crystals of n-type were used. The important property of this system is that it allows the possibility of creating strong electric fields in the gap between the semiconductors. When the applied voltage between semiconductor plates is several volts and the thickness of the gap is in the micrometer range, the electric-field intensities in the gap become quite high and reach the order of 10 kV cm−1 or more. The gap between the cleaved GaAs crystal was filled with liquid DMSO. The focused Gaussian beam with a diameter of 10 µm, of the semiconductor laser with wavelength λ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.65 µm and 5 mW power was incident on the CGCS. The thickness of a gap was varied from 1.5 µm to 3.5 µm, and overall length of the CGCS equals to 0.35 mm. As the length of gap significantly exceeds its thickness,
the field inhomogeneities can be neglected. The focus of lens with focal length F
0.65 µm and 5 mW power was incident on the CGCS. The thickness of a gap was varied from 1.5 µm to 3.5 µm, and overall length of the CGCS equals to 0.35 mm. As the length of gap significantly exceeds its thickness,
the field inhomogeneities can be neglected. The focus of lens with focal length F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 m is located 5 mm behind the CGCS. A principle diagram of this set up is given in Fig. 1. The exact value of thickness was determined using both a microscope and on the basis of the arrangement of diffraction fringes arising from the outgoing radiation. The relative outgoing power Prel was measured using a photodiode, which was connected to an oscilloscope. The applied voltage was varied from 0 to 20 V. The estimated error for Prel
1 m is located 5 mm behind the CGCS. A principle diagram of this set up is given in Fig. 1. The exact value of thickness was determined using both a microscope and on the basis of the arrangement of diffraction fringes arising from the outgoing radiation. The relative outgoing power Prel was measured using a photodiode, which was connected to an oscilloscope. The applied voltage was varied from 0 to 20 V. The estimated error for Prel![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) , including both the increase and decrease of applied voltage was ±0.15. Commercially available DMSO was purified by distillation under reduced pressure.
, including both the increase and decrease of applied voltage was ±0.15. Commercially available DMSO was purified by distillation under reduced pressure.
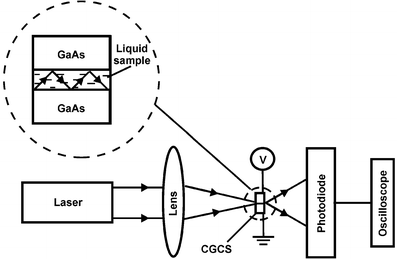 | ||
| Fig. 1 Schematic representation of experimental set up for the measurements of radiation power, which is outgoing from the crystal–gap–crystal structure (CGCS). | ||
Results and discussion
The main aim of the present study was to investigate the effect of a strong electric field on the self-associative structure of pure liquid DMSO. To realize the experiments a hand made setup which included a CGCS and semiconductor laser was constructed. By using a crystal, which can be cleanly cleaved, it is possible to fabricate a structure consisting of two crystals separated from each other by a distance smaller than or of the order of one micrometer. In such a structure the interfaces of the crystals are located exactly parallel to each other because they are formed from one cleaved crystal. This method also gives one the possibility of investigating the liquid in its surface layer. In a previous paper,14 the possibility of creating a plane optical waveguide based on this structure with mobile borders, in which the wave phase can be effectively modulated, has been discussed. The waveguide regime for propagating the radiation through the crystal–gap–crystal structure is realized when the refractive index of the liquid filling the gap exceeds that of the crystal. However, when the refractive index of the crystal exceeds that of the filling liquid, a quasi-waveguide regime of propagation is obtained.The peculiarities of the propagation of the transverse electric modes through this structure under conditions where the refractive index of the crystals (n1) exceeds that of the material filling the clearance (n2) and corresponding equations have been given earlier.14 In this case the wave vector exceeds the coefficient of absorption by one order of magnitude and the formulae we have obtained have a fairly high accuracy and are applicable in the gap and its vicinity. The role of absorption becomes essential when the radiation propagating through the crystal is far from the gap. Only that part of the radiation, which is concentrated in the gap, leaves the structure. As a result one obtains the following formula for the power of the outgoing radiation P(n2):
 | (1) |
The power Prel of the outgoing radiation as a function of the applied voltage U was recorded. The results at t![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C are plotted in Fig. 2. It is clear that the intensity is characterized by a threshold voltage at which it falls sharply with a strong instability of the outgoing intensity. The value of the threshold voltage increases with increase in both the temperature and the thickness of the gap. For DMSO when the thickness (d) of the gap is 2 µm the threshold voltage equals to 9 V, whereas in the case when d
°C are plotted in Fig. 2. It is clear that the intensity is characterized by a threshold voltage at which it falls sharply with a strong instability of the outgoing intensity. The value of the threshold voltage increases with increase in both the temperature and the thickness of the gap. For DMSO when the thickness (d) of the gap is 2 µm the threshold voltage equals to 9 V, whereas in the case when d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 µm, the latter becomes 12 V (this example is illustrated in Fig. 2). When the value of the applied voltage becomes equal to the threshold voltage a jumping change of the outgoing radiation intensity occurs. This transition is continuing for about 3 to 5 min. The discontinuity
is accompanied by strong instability of the outgoing radiation. Moreover, as one can see from Fig. 2 the return to the initial state occurs at a lower value of the applied voltage. Thus, the overall results show hysteresis. When the applied voltage is switched off, the process of restoring the system to its initial conditions lasts about 10 min and also is accompanied by instability of the transmitted radiation.
2.5 µm, the latter becomes 12 V (this example is illustrated in Fig. 2). When the value of the applied voltage becomes equal to the threshold voltage a jumping change of the outgoing radiation intensity occurs. This transition is continuing for about 3 to 5 min. The discontinuity
is accompanied by strong instability of the outgoing radiation. Moreover, as one can see from Fig. 2 the return to the initial state occurs at a lower value of the applied voltage. Thus, the overall results show hysteresis. When the applied voltage is switched off, the process of restoring the system to its initial conditions lasts about 10 min and also is accompanied by instability of the transmitted radiation.
 | ||
Fig. 2 Relative outgoing laser light powers against voltage with the crystal–gap–crystal structure filled with liquid DMSO:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × ×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – show the values in the direction of an increase of the voltage; ○
– show the values in the direction of a decrease the voltage. – show the values in the direction of an increase of the voltage; ○
– show the values in the direction of a decrease the voltage. | ||
It should be noted that the time duration of discontinuous transition strongly depends upon applied voltage, e.g. when the latter is 1.5 times more than the threshold voltage this process occurs for several seconds only. Obviously, this phenomenon due to fluctuation processes and then the time scale could be changed in a wider range.
Analogous phenomena have been observed when the clearance is filled with other polar liquids such as water, ethanol and N,N-dimethylformamide (DMFA). On the other hand, in the case of a non-polar liquid such as benzene, a sharp fall of the output radiation was not detected when the voltage across the gap was changed. In this respect it is interesting to note that a value of threshold voltage depends upon the type of polar liquid, and namely its ability to form hydrogen bonds as well as upon the space arrangement of hydrogen bonds, i.e. linear, two or three dimensional hydrogen-bonded network. Indeed, as it follows from the results obtained a sharp fall of the output radiation for DMSO occurs at 12 V, for DMFA at 14 V, whereas for ethanol and water the threshold voltages are 10 V and 7 V correspondingly (in all cases a thickness of the gap is the same and equal to 2.5 µm). These results are in good agreement with the different ability of these compounds to form hydrogen bonds: the threshold voltage is less for ethanol and water. Furthermore as water built-up three-dimensional hydrogen bonds its structural transition due to cooperative association occurs at the lower value of the threshold voltage (7 V), whereas for ethanol, which is built upon two-dimensional hydrogen-bonded structures, a threshold voltage is somewhat more (10 V).
Conclusion
We interpret the appearance of this phenomenon to be a result of the fact that the charged surfaces of the semiconductors on either side of the gap are conducive to the formation of quite large associated structures in the polar liquid. According to ref. 11–13 the self-association of DMSO molecules on solid surfaces occurs via dipole–dipole interactions. We suggest that at the threshold voltage the formation of bigger aggregates from smaller ones take place. The refractive index of the associates is significantly different from that of the “normal” liquid, and starting from approximately 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 V cm−1
(E
104 V cm−1
(E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V/d) a change of refractive indexes takes place. As a result the scattered laser light after experiencing a quasi-waveguide regime of propagation is partially disordered
so that the outgoing intensity falls. It is interesting to note that this situation is analogous to the enhancement of scattered light at low temperatures due to an increase in the fluctuation concentration of the associates.1
V/d) a change of refractive indexes takes place. As a result the scattered laser light after experiencing a quasi-waveguide regime of propagation is partially disordered
so that the outgoing intensity falls. It is interesting to note that this situation is analogous to the enhancement of scattered light at low temperatures due to an increase in the fluctuation concentration of the associates.1
Acknowledgements
This work has been supported by International Science and Technological Center (ISTC) project # A-199.References
- S. A. Markarian, K. R. Grigorian and L. K. Simonian, J. Chem. Soc., Faraday Trans. 1, 1987, 83, 1189 RSC.
- S. Itoh and H. Ohtaki, Z. Naturforsch., A, 1987, 42, 858 Search PubMed.
- H. Bertagnolli, E. Schultz and P. Chieux, Ber.Bunsen-Ges Phys. Chem., 1989, 93, 88 Search PubMed.
- A. Luzar, A. K. Soper and D. Chandler, J. Chem. Phys., 1993, 99, 6836 CrossRef CAS.
- B. G. Rao and U. C. Singh, J. Am. Chem. Soc., 1990, 112, 3803 CrossRef CAS.
- I. I. Vaisman and M. L. Berkowitz, J. Am. Chem. Soc., 1992, 114, 7889 CrossRef CAS.
- W. R. Fawcett and A. A. Kloss, J. Chem. Soc., Faraday Trans., 1996, 92, 3333 RSC.
- C. Czeslik and J. Jonas, J. Phys. Chem. A, 1999, 103, 3222 CrossRef CAS.
- S. A. Markarian and M. Stockhausen, Z. Naturforsch., A, 2000, 55, 667 Search PubMed.
- G. Fini and P. Mirone, Spectrochim. Acta, Part A, 1976, 32, 625 CrossRef.
- A. Shen and J. E. Pemberton, J. Electroanal. Chem., 1999, 479, 32 CrossRef CAS.
- S. K. Si and A. A. Gewirth, J. Phys. Chem. B, 2000, 104, 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 775 CrossRef CAS.
775 CrossRef CAS. - C. Czeslik, Y. J. Kim and J. Jonas, J. Chem. Phys., 1999, 111, 9739 CrossRef CAS.
- V. M. Aroutiounian, N. L. Markaryan and Kh. V. Nerkararian, Sens. Actuators, A, 1998, 65, 123 Search PubMed.
| This journal is © the Owner Societies 2002 |
