DOI:
10.1039/B110466G
(Paper)
CrystEngComm, 2002,
4, 1-6
Preparation, X-ray crystal structures and properties of α-(BEDT-TTF)2[FeIII(phen)(NCS)4]·2CH2Cl2 and (BEDT-TTF)[CrIII(isoq)2(NCS)4] (phen = 1,10-phenanthroline; isoq = isoquinoline)
Received
16th November 2001
, Accepted 14th December 2001
Abstract
This paper reports the preparation, X-ray crystal structure, conducting and magnetic properties of α-(BEDT-TTF)2[FeIII(phen)(NCS)4]
(1) [BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene, phen = 1,10-phenanthroline] together with the crystal structure of (BEDT-TTF)[CrIII(isoq)2(NCS)4]
(2) (isoq = isoquinoline) for which the physical properties have been reported previously. Compound 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a
= 12.1528(1), b
= 16.8269(3), c
= 27.0703(4)
Å, α
= 95.726(1), β
=
95.834(1), γ
= 108.080(1)°, Z
= 4, R
= 0.0610 for 9764 reflections with I > 2σ(I); 2 crystallizes in the monoclinic space group P21/c
(no. 14), a
=10.623(5), b
= 14.656(8), c
= 12.701(7)
Å, β
= 100.19(2)°, Z
= 2, R
= 0.0737 for 2747 reflections with I > 2σ(I). The magnetic and transport properties have shown that compound 1 is a paramagnetic semiconductor with σ300 K
= 2.2 × 10−2
Ω−1 cm−1.
(no. 2), a
= 12.1528(1), b
= 16.8269(3), c
= 27.0703(4)
Å, α
= 95.726(1), β
=
95.834(1), γ
= 108.080(1)°, Z
= 4, R
= 0.0610 for 9764 reflections with I > 2σ(I); 2 crystallizes in the monoclinic space group P21/c
(no. 14), a
=10.623(5), b
= 14.656(8), c
= 12.701(7)
Å, β
= 100.19(2)°, Z
= 2, R
= 0.0737 for 2747 reflections with I > 2σ(I). The magnetic and transport properties have shown that compound 1 is a paramagnetic semiconductor with σ300 K
= 2.2 × 10−2
Ω−1 cm−1.
Introduction
Radical cation salts and charge transfer complexes based on tetrathiafulvalene (TTF) and its derivatives constitute a wide class of organic materials with transport properties ranging from insulating to superconducting.1 The relative arrangement of the molecules in the solid state was found to be a crucial parameter in determining the properties of the target compound.2 Investigations of organic/inorganic hybrid molecular materials combining conducting electrons and localized spins are of great interest.3–10 When combining the two properties one of the aims is to obtain long-range magnetic coupling between isolated localized spins of the inorganic networks that contain transition metals (d-electrons)
via the mobile electrons within the organic networks (π-electrons). To satisfy this model, which is based on
the synergy between electrical conductivity and magnetic interactions, two conditions are needed, namely good electrical conductivity and strong interactions between the organic and inorganic networks. In order to establish magnetic and/or structural interactions between the organic and inorganic sublattices, several methods are under investigation. Recently, TTF-derived salts containing [Cr(NCS)4(L)n]−, L = 1,10-phenanthroline (phen) or isoquinoline (isoq), have been reported.10 These salts were designed with the idea that magnetic interactions would be induced by both S⋯S contacts and π-stacking overlap between the paramagnetic anions and organic radicals. Indeed, a large number of the salts showed long-range ferrimagnetic order with Tc values ranging from 4.2 to 8.9 K. Other work in this area is based on interactions
through –N⋯I– contacts11 or covalent bonding between the conducting and the paramagnetic networks.12 Continuing our efforts in this line, we report here the preparation, X-ray crystal structure, transport and magnetic properties of α-(BEDT-TTF)2[FeIII(phen)(NCS)4]
(1) [BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene, phen = 1,10-phenanthroline] as well as the crystal structure of (BEDT-TTF)[CrIII(isoq)2(NCS)4]
(2) (isoq = isoquinoline) for which the physical properties have been reported previously for a microcrystalline sample.10
Experimental
Synthesis
All experiments were conducted under nitrogen or argon. The solvents were distilled before use and the starting reagents were used as received. [Bu4N][Fe(phen)(NCS)4]
(phen = C12H8N2) and [isoqH][Cr(isoq)2(NCS)4]·3H2O (isoq = C9H7N) were prepared following the published methods.10 The stoichiometries of the reported materials were identified by X-ray crystal structure analysis, since the electrochemical technique produces small quantities, unsuitable for elemental analysis.
α-(BEDT-TTF)2[FeIII(phen)(NCS)4]
(1).
Black, slightly elongated, plate crystals were obtained by galvanostatic oxidation of BEDT-TTF (8 mg, 2.1 × 10−2 mmol) over one week, using platinum wire electrodes (∅, 1 mm) and a constant current of ca. 1.0 μA. A solution of [Bu4N][Fe(phen)(NCS)4]
(100 mg, 7 × 10−2 mmol) in CH2Cl2
(20 ml) was used as electrolyte.
(BEDT-TTF)[CrIII(isoq)2(NCS)4]
(2).
Black parallelopipedic crystals were obtained by an identical method as used above: BEDT-TTF (8 mg, 2.1 × 10−2 mmol), platinum wire electrode (∅, 1 mm), I
= 1.0 μA, [isoqH][Cr(isoq)2(NCS)4]·3H2O (100 mg, 6.8 × 10−2 mmol) in CH2Cl2
(20 ml).
Crystallographic data collection and structure determination
Single crystals of compounds 1 and 2 were mounted on an Enraf-Nonius four circle diffractometer equipped with a CCD camera and using a graphite monochromated MoKα radiation source (λ
= 0.71073 Å). Data collection was performed at room temperature. No absorption correction was performed and the structures were solved with SHELXS-9713 and refined with the SHELXL-9713 program, by the full-matrix, least squares method on F2.
Collection parameters and crystallographic data are summarized in Table 1.
| Parameter |
1
|
2
|
|
Click b110466g.txt for full crystallographic data (CCDC 165901 and 165902).
R
1
=
Σ||Fo| − |Fc||/Σ|Fo|.
wR
2
= {Σ[w(Fo2
−
Fc2)2]/Σ[w(Fo2)2]}1/2.
|
| Empirical formula |
C37H26Cl2FeN6S20 |
C32H22CrN6S12 |
|
M
|
1322.59 |
927.28 |
| Crystal system |
Triclinic |
Monoclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2)
(no. 2) |
P21/c
(no. 14) |
|
a/Å |
12.1528(1) |
10.623(5) |
|
b/Å |
16.8269(3) |
14.656(8) |
|
c/Å |
27.0703(4) |
12.701(7) |
|
α/° |
95.726(1) |
90 |
|
β/° |
95.834(1) |
100.19(2) |
|
γ/° |
108.080(1) |
90 |
|
V/Å3 |
5184.4(13) |
1946.3(18) |
|
Z
|
4 |
2 |
|
T/K |
293(2) |
120(2) |
|
μ/mm−1 |
1.238 |
0.972 |
| Radiation, λ/Å |
0.71073 |
0.71073 |
| Reflections collected |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 905 905 |
7538 |
| Independent reflections |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691 691 |
4466 |
|
R
1
b
[I > 2σ(I)] |
0.0610 [9764] |
0.0737 [2747] |
|
wR
2
c
|
0.1297 |
0.1899 |
Magnetic and transport measurements
Magnetic studies were carried out on powdered microcrystalline samples enclosed in a gelatin cap. Magnetic susceptibility measurements were performed with a Quantum Design MPMS-7 SQUID magnetometer, working in dc mode and down to a temperature of 2 K and up to an applied field of 7 T. Diamagnetic corrections were estimated using Pascal's constants. Four- or two-probe dc transport measurments were made using an Oxford Instruments Maglab 2000 equipped with an EP probe, operating from 2 to 350 K. Gold wire electrodes (∅, 0.025 mm) were attached directly to the relevant crystal face using Au paste.
Results and discussion
Crystal structures
Standard ORTEP drawings of the molecular structures, with the atom numbering scheme and 50% thermal ellipsoids, are shown in Figs. 1 and 3a for compounds 1 and 2, respectively.
![ORTEP diagram of compound 1 with 50% probability thermal ellipsoids and the atom numbering scheme. Click image or here to access a 3D representation [molecules C and D show a degree of disorder (see text)].](/image/article/2002/CE/b110466g/b110466g-f1.gif) |
| | Fig. 1 ORTEP diagram of compound 1 with 50% probability thermal ellipsoids and the atom numbering scheme. Click image or 1.htm to access a 3D representation [molecules C and D show a degree of disorder (see text)]. | |
α-(BEDT-TTF)2[FeIII(phen)(NCS)4]
(1).
The asymmetric unit contains two complex anions, four BEDT-TTF molecules, labelled A, B, C and D, and two CH2Cl2 solvent molecules, both in general positions. The ORTEP diagram (Fig. 1) shows that the terminal ethylenic carbon atoms of both C and D molecules are disordered onto two sites each, C51a/C51b, C52a/C52b, C71a/C71b and C72a/C72b. The Fe–N (of NCS) distances [mean value 2.035(4)
Å] are slightly shorter than those to the phenanthroline ligand [mean value 2.188(4)
Å]. The Fe–N–CS angles range from 136.7(5) to 166.7(4)° and deviate slightly from linearity. In common with many
BEDT-TTF charge transfer salts, the crystal packing [Fig. 2(a)] consists of alternate layers of organic and inorganic ions. The structure of the organic layer [Fig. 2(b)] is reminiscent of the well known α-type in the BEDT-TTF series.1 The shortest S⋯S contacts between donors (≤3.60 Å, the sum of S⋯S van der Waals radii) are established between adjacent chains as usually observed in this kind of 2D compound [S9–S39 = 3.472(2)
Å; S23–S25 = 3.489(2)
Å; S10–S38 = 3.478(2)
Å]. The packing in the inorganic layer is similar to that found in (TMTSF)5[CrIII(phen)(NCS)4]2 (TMTSF = tetramethyltetraselenafulvalene).10 Anion–anion interactions are present
in the form of π-stacking between nearest neighbour phen ligands with interplanar separations of 3.51 Å and an interplanar angle of 2.36°. With this arrangement all the NCS ligands are oriented towards the organic layers [Fig. 2(a)] yielding several S⋯S contacts (≤3.80 Å) between the anionic and organic parts: S2–S40 = 3.660(2)
Å; S3–S16 = 3.778(2)
Å; S7–S24 = 3.790(2)
Å; S5–S40 = 3.770(3)
Å.
![(a) Crystal structure of compound 1 viewed along the [010] direction, showing the donor–acceptor layered structure and the π-overlap between the phenanthroline ligands of the anionic sheet. Shortest S⋯S contact values between the anionic and organic parts are shown: S2–S40 3.660(2)
Å; S3–S16 3.778(2)
Å; S7–S24 3.790(2)
Å; S5–S40 3.770(3)
Å. (b)
α-Type of the organic layer. Shortest S⋯S contact measurements between organic parts are shown: S9–S39 3.472(2)
Å; S23–S25 3.489(2)
Å; S10–S38 3.478(2)
Å; S31–S24 3.603(2)
Å; S18–S30 3.612(2)
Å; S26–S16 3.527(2)
Å; S10–S38 3.478(2)
Å; S34–S11 3.508(2)
Å;
S33–S21 3.507(2)
Å.](/image/article/2002/CE/b110466g/b110466g-f2.gif) |
| | Fig. 2 (a) Crystal structure of compound 1 viewed along the [010] direction, showing the donor–acceptor layered structure and the π-overlap between the phenanthroline ligands of the anionic sheet. Shortest S⋯S contact values between the anionic and organic parts are shown: S2–S40 3.660(2)
Å; S3–S16 3.778(2)
Å; S7–S24 3.790(2)
Å; S5–S40 3.770(3)
Å. (b)
α-Type of the organic layer. Shortest S⋯S contact measurements between organic parts are shown: S9–S39 3.472(2)
Å; S23–S25 3.489(2)
Å; S10–S38 3.478(2)
Å; S31–S24 3.603(2)
Å; S18–S30 3.612(2)
Å; S26–S16 3.527(2)
Å; S10–S38 3.478(2)
Å; S34–S11 3.508(2)
Å;
S33–S21 3.507(2)
Å. | |
(BEDT-TTF)[CrIII(isoq)2(NCS)4]
(2).
The two independent components are lying on an inversion center, (1/2 0 1/2) for the anion and (0 0 0) for the BEDT-TTF moiety. The ORTEP diagram shows that the terminal ethylenic carbon atoms are disordered onto two sites, each C15a/C15b and C16a/C16b. The Cr–N (of NCS) distances [mean value 1.983(5)
Å] are slightly shorter than those of the isoquinoline ligand [Cr–N3 = 2.079(5)
Å]. From the 1∶1 stoichiometry, the charge on the BEDT-TTF molecule is assigned as +1. The crystal structure [Fig. 3(b)] can be viewed as alternate organic and inorganic layers in the a direction. The organic layer [Fig. 4(a)] consists of mixed
columns parallel to the c direction of BEDT-TTF radical cations and isoquinoline ligands, the angle between BEDT-TTF and isoquinoline planes being 3.67°. The shortest interplanar separations between adjacent BEDT-TTF–isoquinoline and isoquinoline–isoquinoline units are 3.411(8) and 3.527(8)
Å, respectively. In the projection of the structure in the ac plane [Fig. 4(b)], each BEDT-TTF molecule overlaps with another BEDT-TTF molecule on one side and it overlaps with the isoquinoline ligand on the other side, the shortest intermolecular S⋯S contact between adjacent BEDT-TTF units being S6–S6 = 3.755(4)
Å. In a similar manner, each isoquinoline ligand overlaps on one face with an isoquinoline ligand of a neighbouring anion and with a BEDT-TTF unit on its other face. Short contacts are also present between BEDT-TTF molecules and the anions: S2⋯S3 = 3.496(3)
Å,
and S2⋯S6 = 3.482(3)
Å.
![Compound 2 (a) ORTEP diagram with 50% probability thermal ellipsoids and the atom numbering scheme. (b) Crystal structure viewed along the [001] direction, showing the donor–acceptor layered structure and the π-stacking overlap between the isoquinoline ligands and the BEDT-TTF molecules. Click image or here to access a 3D representation.](/image/article/2002/CE/b110466g/b110466g-f3.gif) |
| | Fig. 3 Compound 2 (a) ORTEP diagram with 50% probability thermal ellipsoids and the atom numbering scheme. (b) Crystal structure viewed along the [001] direction, showing the donor–acceptor layered structure and the π-stacking overlap between the isoquinoline ligands and the BEDT-TTF molecules. Click image or 3b.htm to access a 3D representation. | |
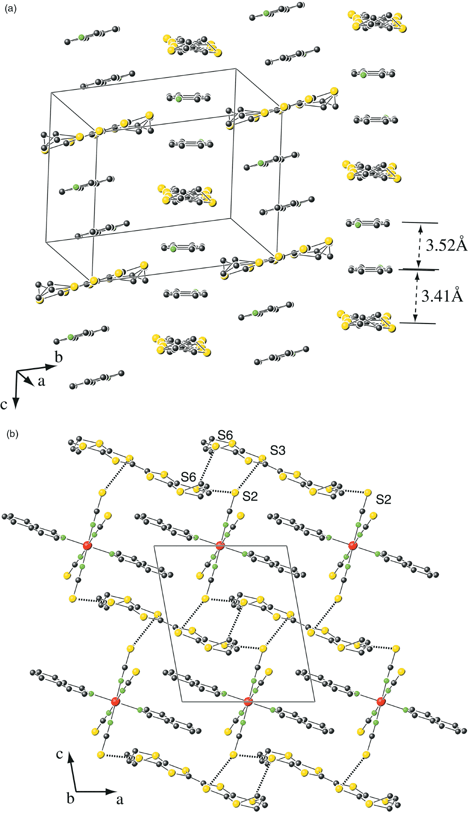 |
| | Fig. 4 Compound 2 (a) projection of the organic layer in the bc plane showing the π-stacking between the isoquinoline ligands and the BEDT-TTF molecule. (b) Projection of the structure in the ac plane showing the shortest S⋯S contacts: S6–S6 = 3.755(4)
Å; S2–S3 = 3.496(3); S2–S6 = 3.482(3)
Å. | |
Magnetic and transport properties
The temperature dependences of the magnetic susceptibility for the title compounds were measured in the temperature range 2–300 K, with an applied field of 1000 G. The ac and dc magnetic susceptibility values of compound 2 were identical to that previously reported for a microcrystaline sample, i.e. it is a bulk ferrimagnet with a Tc of 4.2 K.10 Compound 1 is a simple paramagnet which obeys the Curie–Weiss law with a very small Weiss constant of −0.04 K and a Curie constant of 4.362 emu K mol−1
(a spin only value of 4.377 is expected for high spin Fe3+). Thus the magnetization is dominated by the coordination metal centre.
The transport properties of 1 indicate a highly anisotropic semiconductor. The conductivity versus temperature data (Fig. 5) were measured in three directions with respect to the elongated plates. Perpendicular to the plate, a two probe measurement gave a conductivity at 302 K (σ302 K) of 1.03 × 10−5
Ω−1 cm−1. However, in the plane of the plate, parallel to the longer dimension, the conductivity is three orders of magnitude higher; the high temperature value is σ280 K
= 3.2 × 10−2
Ω−1 cm−1, although the activation energy changes slightly from 0.28 eV at high temperature to 0.17 eV at low temperatures. Parallel to the plane of the short dimension of the plate the conductivity is also relatively high, σ300
K
= 2.2 × 10−2
Ω−1 cm−1. This indicates the two-dimensional nature of 1, as evident from the crystal structure.
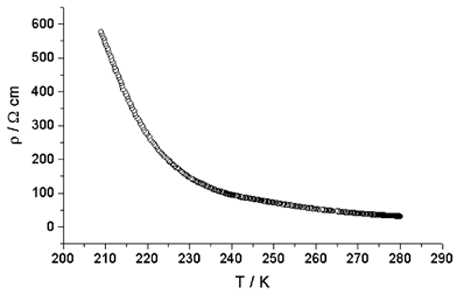 |
| | Fig. 5 Curve of resistivity versus temperature for compound 1 measured in the most conductive direction parallel to the crystal plate. | |
Conclusion
A new charge transfer salt α-(BEDT-TTF)2[FeIII(phen)(NCS)4]
(1) has been obtained and structurally and magnetically characterized. Compound 1 is a paramagnetic semiconductor in which the magnetic characteristics are dominated by the high spin FeIII centre. The absence of long-range magnetic order, as evidenced by compound 2, is most likely due to the absence of π-stacking interactions between the anion and cation components. The transport properties of 1 are highly anisotropic and show two-dimensional characteristics. Additionally, we grew single crystals of the salt (BEDT-TTF)[CrIII(isoq)2(NCS)4]
(2) and determined its crystal structure. The magnetic properties of 2 have already been discussed elsewhere10 and were identical to those of the sample reported here.
Acknowledgements
Financial support from the CNRS, the UK Science and Engineering Research Council and the TMR Program from the European Union (contract ERBFMRXCT98-0181). S. F. thanks the Algerian and French Ministries of Education for a PhD fellowship.
References
-
J. M. Williams, J. R. Ferraro, R. J. Thorn, K. D. Carlson, U. Geiser, H. H. Wang, A. M. Kini and M. H. Whangbo, in Organic Superconductors. Synthesis, Structure, Properties and Theory, ed. R. N. Grimes, Prentice Hall, Englewood Cliffs, New Jersey, 1992 Search PubMed.
- H. Yamochi, K. Tokutaro, N. Matsukawa, G. Saito, M. Takehiko, M. Kusunoki and K. Sakaguch, J. Am. Chem. Soc., 1993, 115, 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 CAS.
319 CAS.
- P. Day, M. Kurmoo, T. Mallah, I. R. Marsden, R. H. Friend, F. L. Pratt, W. Hayes, D. Chasseau, J. Gaultier, G. Bravic and L. Ducasse, J. Am. Chem. Soc., 1992, 114, 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 CAS; A. W. Graham, M. Kurmoo and P. Day, J. Chem. Soc., Chem. Commun., 1995, 2061 RSC.
722 CAS; A. W. Graham, M. Kurmoo and P. Day, J. Chem. Soc., Chem. Commun., 1995, 2061 RSC.
- H. Kobayashi, H. Tomita, T. Naito, A. Kobayashi, F. Sakai, T. Watanabe and P. Cassoux, J. Am. Chem. Soc., 1996, 118, 368 CrossRef CAS; H. Kobayashi, A. Kobayashi and P. Cassoux, Chem. Soc. Rev., 2000, 29, 325 RSC.
- P. Le Maguerès, L. Ouahab, N. Conan, C. J. Gomez-Garcia, P. Delhaès, J. Even and M. Bertault, Solid State Commun., 1996, 97, 27 CrossRef; P. Le Maguerès, L. Ouahab, P. Briard, J. Even, M. Bertault, L. Toupet, J. Ramos, C. J. Gomez-Garcia and P. Delhaès, Mol. Cryst. Liq. Cryst., 1997, 305, 479 Search PubMed.
- T. Enoki, J. L. Yamaura and A. Miyazaki, Bull. Chem. Soc. Jpn., 1997, 70, 2005 CAS.
- L. Ouahab, Chem. Mater., 1997, 9, 1909 CrossRef CAS.
- C. J. Gomez-Garcia, L. Ouahab, C. Gimenez-Saiz, S. Triki, E. Coronado and P. Delhaès, Angew. Chem., Int. Ed. Engl., 1994, 33, 223 CrossRef.
- E. Coronado, J. R. Galan-Mascaros, C. J. Gomez-Garcia and V. N. Laukhin, Nature, 2000, 408, 447 CrossRef CAS.
- S. S. Turner, C. Michaut, S. Durot, D. Day, T. Gelbrich and M. B. Hursthouse, J. Chem. Soc., Dalton Trans., 2000, 905 RSC; S. S. Turner, D. Le Pevelen, D. Day and K. Prout, J. Chem. Soc., Dalton Trans., 2000, 2739 RSC.
- D. Thoyon, K. Okabe, T. Imakubo, S. Golhen, A. Miyazaki, T. Enoki and L. Ouahab, Mol. Cryst. Liq. Cryst., 2001, in press Search PubMed.
- F. Iwahori, S. Golhen, L. Ouahab and R. Carlier, Inorg. Chem., 2001, 40, 6541 CrossRef CAS.
-
G. M. Sheldrick, SHELX-97, Program for the Refinement of Crystal Structures, University of Göttingen, Germany, 1997.
|
| This journal is © The Royal Society of Chemistry 2002 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a
= 12.1528(1), b
= 16.8269(3), c
= 27.0703(4)
Å, α
= 95.726(1), β
=
95.834(1), γ
= 108.080(1)°, Z
= 4, R
= 0.0610 for 9764 reflections with I > 2σ(I); 2 crystallizes in the monoclinic space group P21/c
(no. 14), a
=10.623(5), b
= 14.656(8), c
= 12.701(7)
Å, β
= 100.19(2)°, Z
= 2, R
= 0.0737 for 2747 reflections with I > 2σ(I). The magnetic and transport properties have shown that compound 1 is a paramagnetic semiconductor with σ300 K
= 2.2 × 10−2
Ω−1 cm−1.
(no. 2), a
= 12.1528(1), b
= 16.8269(3), c
= 27.0703(4)
Å, α
= 95.726(1), β
=
95.834(1), γ
= 108.080(1)°, Z
= 4, R
= 0.0610 for 9764 reflections with I > 2σ(I); 2 crystallizes in the monoclinic space group P21/c
(no. 14), a
=10.623(5), b
= 14.656(8), c
= 12.701(7)
Å, β
= 100.19(2)°, Z
= 2, R
= 0.0737 for 2747 reflections with I > 2σ(I). The magnetic and transport properties have shown that compound 1 is a paramagnetic semiconductor with σ300 K
= 2.2 × 10−2
Ω−1 cm−1.![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2)
(no. 2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 905
905![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691
691![ORTEP diagram of compound 1 with 50% probability thermal ellipsoids and the atom numbering scheme. Click image or here to access a 3D representation [molecules C and D show a degree of disorder (see text)].](/image/article/2002/CE/b110466g/b110466g-f1.gif)
![(a) Crystal structure of compound 1 viewed along the [010] direction, showing the donor–acceptor layered structure and the π-overlap between the phenanthroline ligands of the anionic sheet. Shortest S⋯S contact values between the anionic and organic parts are shown: S2–S40 3.660(2)
Å; S3–S16 3.778(2)
Å; S7–S24 3.790(2)
Å; S5–S40 3.770(3)
Å. (b)
α-Type of the organic layer. Shortest S⋯S contact measurements between organic parts are shown: S9–S39 3.472(2)
Å; S23–S25 3.489(2)
Å; S10–S38 3.478(2)
Å; S31–S24 3.603(2)
Å; S18–S30 3.612(2)
Å; S26–S16 3.527(2)
Å; S10–S38 3.478(2)
Å; S34–S11 3.508(2)
Å;
S33–S21 3.507(2)
Å.](/image/article/2002/CE/b110466g/b110466g-f2.gif)
![Compound 2 (a) ORTEP diagram with 50% probability thermal ellipsoids and the atom numbering scheme. (b) Crystal structure viewed along the [001] direction, showing the donor–acceptor layered structure and the π-stacking overlap between the isoquinoline ligands and the BEDT-TTF molecules. Click image or here to access a 3D representation.](/image/article/2002/CE/b110466g/b110466g-f3.gif)


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 CAS.
319 CAS.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 CAS; A. W. Graham, M. Kurmoo and P. Day, J. Chem. Soc., Chem. Commun., 1995, 2061 RSC.
722 CAS; A. W. Graham, M. Kurmoo and P. Day, J. Chem. Soc., Chem. Commun., 1995, 2061 RSC.