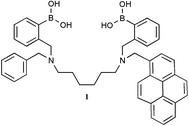
A modular electrochemical sensor for saccharides†
Susumu
Arimori
,
Shin
Ushiroda
,
Laurence M.
Peter
,
A. Toby A.
Jenkins
and
Tony D.
James
*
Department of Chemistry, University of Bath, Bath, UK BA2 7AY. E-mail: T.D.James@bath.ac.uk
First published on 18th September 2002
Abstract
A modular electrochemical saccharide sensor using ferrocene has been prepared which contains two boronic acid receptor groups and hexamethylene linker.
Much recent attention has been paid to the development of synthetic molecular receptors with the ability to recognise neutral organic species, including saccharides.1–3 Boronic acid receptors for saccharides have attracted considerable interest.2,3 Boronic acids are known to bind saccharides via covalent interactions in aqueous basic media. The most common interaction is with cis-1,2- or 1,3-diols of saccharides to form five- or six-membered rings respectively. The interaction of a boronic acid (Lewis acid) and neighbouring tertiary amine (Lewis base) is strengthened on saccharide binding.2,3
Over the last few years we have been interested in developing new sensors selective for saccharides employing a modular approach. The basic idea was to break a sensor into three components; receptor units, linker units, and ‘read-out’ units. The approach can be illustrated by describing the D-glucose selective fluorescence sensor 1 which contains two boronic acid units (receptors), hexamethylene unit (D-glucose selective linker), and pyrene unit (fluorophore-‘read-out’).4,5 Using compound 1 as a model any new saccharide selective sensor requires at least two boronic acid units, one linker unit, and a ‘read-out’ unit. Two or more boronic acid units as with sensor 1 are required because only through two points binding can saccharide selectivity be controlled. One ‘read-out’ unit is required as with sensor 1 to report on saccharide binding. Also a linker is required and the choice of linker is very important since it will determine the selectivity of the sensor. With compound 1, a hexamethylene linker results in D-glucose selectivity.4,5 Following the criteria given above and the requirements for electrochemical detection,6 we designed the electrochemical saccharide sensor 2 with two boronic acid units (saccharide selectivity), one ferrocene unit and a hexamethylene linker unit (D-glucose selectivity).
Electrochemical detection of saccharides by enzymatic decomposition of saccharides is well known.7 The development of boronic acid based electro-active saccharide receptors could provide selectivity for a range of saccharides. Chiral ferroceneboronic acid derivatives have been synthesized and tested for chiral electrochemical detection of monosaccharides.8 A recent paper by Moore and Wayner has explored the redox switching of carbohydrate binding with commercial ferrocene boronic acid.9 With both of the above systems, D-fructose selectivity was observed which is the inherent selectivity for all monoboronic acids.10 Also, for these two systems the observed binding with D-glucose is very weak, the calculated stability constants are <20 dm3 mol−1.
With this research we set out to design a diboronic acid system which could be used to control saccharide selectivity through two-point binding. Synthesis of 2 and reference compounds 3,114 and 5 was achieved according to Scheme 1 from readily available starting materials.‡
 | ||
| Scheme 1 Synthesis of 2, 3, 4 and 5. Reagents (yields): i) benzaldehyde, p-toluenesulfonic acid, THF/EtOH: ii) NaBH4, 79% (2 steps): iii) ferrocenecalboxaldehyde, THF/MeOH: iv) NaBH4, 76% (2 steps): v) 2-(2-bromobenzyl)-1,3-dioxaborinane, K2CO3, MeCN 69%: vi) methylamine, THF/MeOH: vii) NaBH4, 74% (5), 55% (6) (2 steps): viii) 2-(2-bromobenzyl)-1,3-dioxaborinane, K2CO3, MeCN 42% (3), 10% (4). | ||
Differential pulse voltammograms (DPV) were recorded for reference compound 5 (5.0 × 10−5 mol dm−3),§ a 1∶1 mixture of compound 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (5.0 × 10−5 mol dm−3)§ and 2 (5.0 × 10−5 mol dm−3) (Fig. 1) with D-glucose (0–0.1 mol dm−3) in aqueous methanolic buffer solution [52.1 wt% methanol at pH 8.21 (KCl, 0.01000 mol dm−3; KH2PO4, 0.002752 mol dm−3; Na2HPO4, 0.002757 mol dm−3)]. DPV removes the effect of electrode capacitive charging, resulting in measurement of only the Faradaic proceeses and hence much higher signals than conventional voltammetries.12 The DPV were recorded with an Ecochemie μ-Autolab potentiostat, using a single-compartment cell fitted with a glassy carbon electrode for working electrode (0.28 cm2), a platinum plate for counter electrode, and a Ag/AgCl reference electrode. From Fig. 1 it can be seen that the oxidation potential of the ferrocene becomes more anodic on binding with saccharides. The interaction of the boronic acid and neighbouring amine is strengthened on saccharide binding,2,3 thereby, reducing the electron density on the neighbouring amine. This in turn destabilises the ferrocenium ion at higher concentrations of saccharides, resulting in a more anodic ferrocene oxidation overpotential (Scheme 2).
4 (5.0 × 10−5 mol dm−3)§ and 2 (5.0 × 10−5 mol dm−3) (Fig. 1) with D-glucose (0–0.1 mol dm−3) in aqueous methanolic buffer solution [52.1 wt% methanol at pH 8.21 (KCl, 0.01000 mol dm−3; KH2PO4, 0.002752 mol dm−3; Na2HPO4, 0.002757 mol dm−3)]. DPV removes the effect of electrode capacitive charging, resulting in measurement of only the Faradaic proceeses and hence much higher signals than conventional voltammetries.12 The DPV were recorded with an Ecochemie μ-Autolab potentiostat, using a single-compartment cell fitted with a glassy carbon electrode for working electrode (0.28 cm2), a platinum plate for counter electrode, and a Ag/AgCl reference electrode. From Fig. 1 it can be seen that the oxidation potential of the ferrocene becomes more anodic on binding with saccharides. The interaction of the boronic acid and neighbouring amine is strengthened on saccharide binding,2,3 thereby, reducing the electron density on the neighbouring amine. This in turn destabilises the ferrocenium ion at higher concentrations of saccharides, resulting in a more anodic ferrocene oxidation overpotential (Scheme 2).
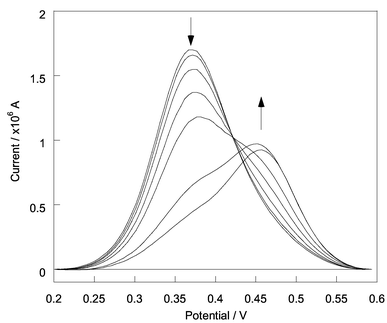 | ||
| Fig. 1 Differential pulse voltammograms of 2 (5.0 × 10−5 mol dm−3) with different concentration of D-glucose (0, 1.11 × 10−4, 3.89 × 10−4 6.66 × 10−4 1.04 × 10−3, 1.03 × 10−2, 1.02 × 10−1 mol dm−3) at pH 8.21 in 52.1 wt% methanol. The area of the glassy carbon electrode is 0.28 cm2. Voltammetric parameters are as follows: scan rate, 20 mV s−1; modulation time, 50 ms; interval time, 500 ms; step potential, 5.1 mV; modulation amplitude 25.05 mV. The lack of an isobestic point is attributed to viscosity effects.‡ | ||
 | ||
| Scheme 2 | ||
The stability constants (K) of electrochemical sensors 2 and the 1∶1 mixture of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were calculated by fitting the current intensity at 0.357 V vs. saccharide concentration and are given in Table 1.13,14
4 were calculated by fitting the current intensity at 0.357 V vs. saccharide concentration and are given in Table 1.13,14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4
4
| K/mol−1 dm3 at 25 °C | |||
|---|---|---|---|
| Saccharide | 2 | 3+4 |
2/3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4 4 |
| D-Glucose | 684 ± 54 (0.99) | 17 ± 2 (0.99) | 40 |
| D-Fructose | 1478 ± 72 (1.00) | 362 ± 5 (1.00) | 4 |
| D-Galactose | 782 ± 72 (0.99) | 47 ± 2 (1.00) | 17 |
| D-Mannose | 149 ± 9 (1.00) | 54 ± 8 (0.99) | 3 |
The relative stability constants of the diboronic acid 2 relative to the 1∶1 mixture of monoboronic acids 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 are given in Table 1. A 1∶1 mixture of monoboronic acids 3
4 are given in Table 1. A 1∶1 mixture of monoboronic acids 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was used as the reference to alow direct comparison with the diboronic acid system 2. Using a 1∶1 mixture ensures that the same concentration of both ferrocene and boronic acid groups are present in both cases.
4 was used as the reference to alow direct comparison with the diboronic acid system 2. Using a 1∶1 mixture ensures that the same concentration of both ferrocene and boronic acid groups are present in both cases.
Although the highest binding constant for compound 2 is with D-fructose, cooperative binding of the two boronic acid groups is clearly observed as illustrated by the stability constant differences between the mono- and di-boronic acid compounds (sensors 2 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, respectively). In particular the stability constant K of diboronic acid sensor 2 with D-glucose was 40 times greater than with monoboronic sensor 3
4, respectively). In particular the stability constant K of diboronic acid sensor 2 with D-glucose was 40 times greater than with monoboronic sensor 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4, whereas the stability constant K of diboronic acid sensor 2 with D-fructose was only 4 times stronger than monoboronic sensor 3
4, whereas the stability constant K of diboronic acid sensor 2 with D-fructose was only 4 times stronger than monoboronic sensor 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4. These results are not surprising since it is well known that D-glucose easily forms 1∶1 cyclic complexes with di-boronic acids, whereas D-fructose tends to form 2∶1 acyclic complexes with di-boronic acids.2,3
4. These results are not surprising since it is well known that D-glucose easily forms 1∶1 cyclic complexes with di-boronic acids, whereas D-fructose tends to form 2∶1 acyclic complexes with di-boronic acids.2,3
In conclusion, we have shown that it is possible to prepare an electrochemical sensor 2 with enhanced D-glucose (40 fold) and D-galactose (17 fold) binding employing simple building blocks and using a modular approach. We believe that these results could be applied in the development of new saccharide selective electrochemical sensors. Our ongoing research in directed towards new modular electrochemical sensors with different linkers and electroactive units.
T. D. J. wishes to acknowledge the Royal Society, the EPSRC, and Beckman-Coulter for support. S. A. wishes to acknowledge Beckman Coulter for support through the award of a Postdoctoral Fellowship. S. U. wishes to acknowledge the EPSRC for support through the award of an EPSRC studentship. A. T. A. J. wishes to acknowledge the Nuffield foundation for an equipment grant. We wish to thank Dr Noel W. Duffy for helpful advice and discussions. We would also like to acknowledge the support of the University of Bath.
Notes and references
- A. P. Davis and R. S. Wareham, Angew. Chem., Int. Ed., 1999, 38, 2979 CAS.
- J. H. Hartley, T. D. James and C. J. Ward, J. Chem. Soc., Perkin Trans. 1, 2000, 3155 RSC.
- T. D. James and S. Shinkai, Top. Curr. Chem., 2002, 218, 159 Search PubMed.
- S. Arimori, M. L. Bell, C. S. Oh, K. A. Frimat and T. D. James, Chem. Commun., 2001, 1836 RSC.
- S. Arimori, M. L. Bell, C. S. Oh, K. A. Frimat and T. D. James, J. Chem. Soc., Perkin Trans. 1, 2002, 802 Search PubMed.
- P. D. Beer, P. A. Gale and G. Z. Chen, J. Chem. Soc., Dalton Trans., 1999, 1897 RSC.
- W. Schuhmann and H.-L. Schmidt, Adv. Biosensors, 1992, 2, 79 Search PubMed.
- A. Ori and S. Shinkai, J. Chem. Soc., Chem. Commun., 1995, 1771 RSC.
- A. N. J. Moore and D. D. M. Wayner, Can. J. Chem.-Rev. Can. Chim., 1999, 77, 681 Search PubMed.
- J. P. Lorand and J. D. Edward, J. Org. Chem., 1959, 24, 769 CrossRef CAS.
- The reference compound 3 has recently been prepared and proposed as a potentially active electrochemical unit by J. C. Norrild and I. Sotofte, J. Chem. Soc., Perkin Trans 2, 2002, 303 Search PubMed.
- A. Bard and L. Faulkner, Electrochemical Methods, 2nd edition, Wiley, New York, 2001 Search PubMed.
- The K values were analysed in KaleidaGraph using nonlinear (Levenberg–Marquardt algorithm) curve fitting. The errors reported are the standard errors obtained from the best fit.
- C. R. Cooper and T. D. James, J. Chem. Soc., Perkin Trans. 1, 2000, 963 RSC.
Footnotes |
† Electronic supplementary information (ESI) available: observed DPV for 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 5. See http://www.rsc.org/suppdata/cc/b2/b207643h/ 4 and 5. See http://www.rsc.org/suppdata/cc/b2/b207643h/ |
‡ Selected data for 2: mp 155–158 ° C (decomp.); m/z (FAB) 1212 ([M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4(3-HOCH2C6H4NO2) 4(3-HOCH2C6H4NO2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4H2O]+, 95%); Found: C, 69.25; H, 7.07; N, 4.12. C38H46B2FeN2O4-H2O+0.05 CHCl3 requires C, 69.21; H, 6.74; N, 4.24%. δH(300 MHz, CDCl3 4H2O]+, 95%); Found: C, 69.25; H, 7.07; N, 4.12. C38H46B2FeN2O4-H2O+0.05 CHCl3 requires C, 69.21; H, 6.74; N, 4.24%. δH(300 MHz, CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD (a few drops), Me4Si): 1.28 (4H, br s, NCC(CH2)2), 1.42 (4H, br s, NC(CH2) 2), 2.28 (2H, t, FcCNCH2), 2.36 (2H, t, NCH2), 3.56 (4H, s, NCH2Ph), 3.58 (2H, s, FcCH2N), 3.78 (2H, s, NCH2Ph), 4.12 and 4.18 (5H, 4H, s each, Fc-H), 7.15–7.38, 7.86 (11H, 2H, m each, Ar-H). δC (75 MHz, CDCl3 CD3OD (a few drops), Me4Si): 1.28 (4H, br s, NCC(CH2)2), 1.42 (4H, br s, NC(CH2) 2), 2.28 (2H, t, FcCNCH2), 2.36 (2H, t, NCH2), 3.56 (4H, s, NCH2Ph), 3.58 (2H, s, FcCH2N), 3.78 (2H, s, NCH2Ph), 4.12 and 4.18 (5H, 4H, s each, Fc-H), 7.15–7.38, 7.86 (11H, 2H, m each, Ar-H). δC (75 MHz, CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD (a few drops), Me4Si): 22.7, 24.7, 25.6, 27.0, 31.6, 51.2, 52.0, 57.1, 60.4, 61.3, 68.4, 68.5, 68.6, 68.9, 70.6, 127.2, 127.3, 127.5, 128.2, 128.5, 129.6, 129.7, 129.9, 130.6, 130.9, 136.2, 136.5, 141.7, 141.8. CD3OD (a few drops), Me4Si): 22.7, 24.7, 25.6, 27.0, 31.6, 51.2, 52.0, 57.1, 60.4, 61.3, 68.4, 68.5, 68.6, 68.9, 70.6, 127.2, 127.3, 127.5, 128.2, 128.5, 129.6, 129.7, 129.9, 130.6, 130.9, 136.2, 136.5, 141.7, 141.8. |
§ The observed DPV for 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4 and 5 are available as ESI.† A decrease in current intensity with increasing D-glucose concentration was observed. Since reference compound 5 can not bind with D-glucose, the observed decrease in current intensity is due to a change in viscosity of the solution with added D-glucose. With compound 5 for concentrations of D-glucose greater than 0.1 mol dm−3 the current dramatically decreases due to high solution viscosity. Therefore, the ferrocene boronic acid sensors were titrated until a concentration of 0.1 mol dm−3 saccharide. 4 and 5 are available as ESI.† A decrease in current intensity with increasing D-glucose concentration was observed. Since reference compound 5 can not bind with D-glucose, the observed decrease in current intensity is due to a change in viscosity of the solution with added D-glucose. With compound 5 for concentrations of D-glucose greater than 0.1 mol dm−3 the current dramatically decreases due to high solution viscosity. Therefore, the ferrocene boronic acid sensors were titrated until a concentration of 0.1 mol dm−3 saccharide. |
| This journal is © The Royal Society of Chemistry 2002 |