High yield conversion of methane to methyl bisulfate catalyzed by iodine cations
Roy A.
Periana
*a,
Oleg
Mirinov
a,
Douglas J.
Taube
b and
Scott
Gamble
b
aUniversity of Southern California, Department of Chemistry, Loker Hydrocarbon Institute, Los Angeles, CA 90089. E-mail: rperiana@usc.edu; Fax: 213-821-2656; Tel: 213-821-2035
bCatalytica Advanced Technologies, Inc, 430 Ferguson Drive, Mountain View, CA 94043
First published on 18th September 2002
Abstract
Iodine in 2% oleum is an efficient catalyst for the selective, high yield oxidation of methane to methyl bisulfate.
Recently, several reports of alkane CH activation by homogeneous, cationic metal complexes with poorly coordinating anions in poorly coordinating solvents have been reported.1 The success of this strategy relies on the ease of substitution of the weakly coordinating anion or solvent by the poorly coordinating alkanes. A related strategy that lends itself well to net CH functionalization via the CH activation reaction is to generate such poorly coordinated, redox-active, cationic species by use of strongly acidic, oxidizing solvents.
Attesting to the utility of this strategy, to date, the highest reported yields and selectivities for the low temperature oxidation of methane to methanol are with cationic catalysts in sulfuric acid solvent.2 A key requirement for working in such strongly acidic, poorly coordinating, oxidizing media is that the catalysts be stable.
Herein we report that 1–10 mM elemental iodine dissolved in sulfuric acid containing 2–3% SO3 (oleum) generates a stable, active species that at 165–220 °C catalyzes the functionalization of methane (500 psig) to methyl bisulfate, eqn. (1).3 Concentrations of methyl bisulfate of up to 1M, 45% yields (based on methane) at >90% selectivity, volumetric productivities of ∼10−7 mol per cc.s, turn-over numbers of 300 and turn-over-frequencies of 3 × 10−2 s−1 have been observed. Iodine is required for the reaction as no methyl bisulfate is formed in the absence of added iodine, Table 1, # 7. Use of enriched 13C methane and 1H, 13C NMR and HPLC analyses of the crude reaction mixtures confirmed that methyl bisulfate is the only liquid-phase product generated from methane; no methanol, methyl iodide, methane sulfonic acid (CH3SO3H) or other liquid phase produced were observed. Only SO2 and very low levels (<1% based on added CH4) of CO2 were observed in the gas phase. Carbon mass-balances of >95% based on unreacted methane and methyl bisulfate confirmed the high reaction selectivity and yield. The reaction rates and selectivities are reproducible and first order dependence on both methane (Table 1, # 4 and 8) and iodine (Table 1, # 4–7) are observed. The reaction rate is also strongly dependent on the concentration of SO3 in the sulfuric acid solvent and does not proceed in <98% H2SO4 where free SO3 is not present, Table 1, # 1–4.4 Reactions of higher alkanes with oleum catalyzed by iodine were briefly examined but while oxidation to esters was observed, the reactions are much less selective than with methane. Evidently, the increased rates of proton catalyzed side reactions with these higher alkanes make them unsuitable substrates in oleum.
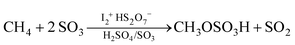 | (1) |
| # | SO3 Wt | X2 (mM) | CH4 psig | % CH4 Conv | TOF s−1 | [CH3Xb] mM, % sel |
|---|---|---|---|---|---|---|
| a Reaction time and temperature: 60 min at 195 °C. b 96% H2SO4. c X = OSO3H. d 50 psig O2 added. e 10 mM K2S2O8 added. | ||||||
| 1 | 0c | I2 (5) | 500 | 0 | 0 | 0, 0 |
| 2 | 0.5 | I2 (5) | 500 | — | 0.003 | 54, 95 |
| 3 | 1 | I2 (5) | 500 | 10 | 0.012 | 218, 95 |
| 4 | 2.5 | I2 (5) | 500 | 30 | 0.033 | 600, 95 |
| 5 | 2.5 | I2 (10) | 500 | 53 | 0.03 | 1050, 95 |
| 6 | 2.5 | I2 (1) | 500 | 5 | 0.035 | 95, 95 |
| 7 | 2.5 | none | 500 | 0 | 0 | 0, 0 |
| 8 | 2.5 | I2 (5) | 200 | 10 | 0.01 | 205,95 |
| 9 | 2.5 | Br2 (10) | 500 | 20 | — | 240. 50 |
| 10 | 2.5 | Cl2 (10) | 500 | 25 | — | 150, 30 |
| 11 | 2.5 | I2 (5) | 500d | 31 | 0.033 | 610, 95 |
| 12 | 2.5 | I2 (5) | 500e | 28 | 0.025 | 575, 94 |
Other researchers have reported on the use of sulfuric acid and other strong acid solvents for conversion of methane but in these cases, the product yields, selectivities and volumetric productivities were significantly lower and relatively expensive oxidants, such as persulfate were utilized.4,5 Given the simplicity of the catalyst and high reaction efficiency and selectivity, it is of interest to provide some information on the reaction mechanism.
Two key considerations of our work were to determine the nature of the species that reacts with methane and whether free radicals are involved. It is well known that methane can react with super-acids such as HF∶SbF5via the formation of CH5+. 6 Consequently, it is possible that added iodine catalyses the oxidation of CH5+ species by SO3 to generate methyl bisulfate. To investigate this we examined the extent of deuterium incorporation into methane as well as methyl bisulfate in the presence and absence of added iodine when the reaction was carried out in D2SO4/SO3. Consistent with earlier reports,6 we find extensive, ∼50%, deuterium exchange into methane even at short reaction times (30 min) in the absence of iodine. Interestingly, the extent of deuterium exchange between the D2SO4/SO3 solvent and gas-phase methane is suppressed when iodine is added presumably due to a net decrease in solvent acidity on addition of iodine and the known6 strong dependence of the exchange on solvent acidity. More significantly, the methyl bisulfate produced (which as noted above is only formed in the presence of added iodine) shows only low levels (<5%) of deuterium incorporation under the same conditions. This observation makes it unlikely that the methyl bisulfate is formed from iodine catalyzed oxidation of a putative CDH4+ intermediate as statistically, (ignoring isotope effects) this should lead to the formation of a minimum of ∼20% CDH2OSO3H.7
It has been reported that S82+ cations reacts slowly with methane at room temperature in liquid SO2 to generate low yields of methane thiol although no catalysis or mechanistic results were reported.8 Gillespie has shown that the characteristic bright blue color formed on dissolution of iodine in oleum is due to the formation of the paramagnetic species I2+.9 The reported disproportionation of I2+ in oleum with decreasing SO39 to form the more stable, poly-iodo cations, I3+ or I4+, coupled with our observations of the strong dependence of the methane oxidation reaction on SO3 concentration is consistent with either I2+ or possibly I+ (which could be formed at the elevated reaction temperatures although it has never been synthesized) being the reactive species. Other possible reactive species are iodyl sulfate (IO2+HSO4−),10 iodosyl sulfate (IO+HSO4−)10 and I(HSO4)311 as these are also reported to be formed on oxidation of iodine in sulfuric acid.
To determine which of these species could be the active catalyst we explicitly examined the stoichiometric reactions of methane at 50 °C with independently synthesized samples of I2+ [Sb2F11]−, IOHSO4, IO2HSO4 and I(HSO4)3 in oleum and SO2 solvents. Significantly, only the I2+[Sb2F11]− shows stoichiometric reactions under these mild conditions. Thus, reaction of 15 ml of 100 mM I2+Sb2F11− (1.5 mmols) with excess methane (30 mmols) at 50 °C for 1 h in 2.5 wt% oleum led to 30% yield of methyl bisulfate based on the added I2+Sb2F11−. Importantly, no reaction is observed at these mild conditions if the I2+Sb2F11− is replaced with 100 mmol of either elemental iodine, IOHSO4, IO2HSO4 or I(HSO4)3. Repeating the reaction with 100 mmols of I2+[Sb2F11]− in 96% sulfuric acid also gave no methyl bisulfate and is consistent with reported instability of I2+ species in SO3-free sulfuric acid solutions.9 We also examined the reaction of SO2 solutions of I2+Sb2F11− with methane at 35 °C as, unlike oleum, this solvent is not oxidizing and is unlikely to oxidize the I2+ to higher oxidation state species. As was reported for the case of S82+,8 there is a clear reaction with methane as the blue color of the I2+Sb2F11− is bleached on exposure to 500 psig of methane after 10 h. However, the reaction is not as clean as in oleum and NMR analysis shows that several methyl products, among them (CH3)2I+ and CH3F, are formed.
These observations are consistent with I2+ (or I+) species as the active catalyst but do not indicate whether free radical mechanisms are involved. While the free radical reaction of atomic iodine with methane can be discounted,12 free radical reactions are plausible with the stronger oxidants I2+, IOHSO4, IO2HSO4 or I(HSO4)3. However, given the high reaction yield and selectivity as well as reproducible, first order kinetics with respect to both methane and iodine, we are biased toward a non-free radical pathway. This bias is strengthened by the observation that added oxygen gas or K2S2O8 (Table 1, #11) has no affect on the reaction rate or selectivity. Oxygen and persulfate are known radical scavengers and initiators, respectively, and these species could be expected to lead to changes in rate or selectivity. We also investigated elemental bromine and chlorine as methane oxidation catalysts in 2–3% oleum. In both of these cases, both the reaction rates and selectivities to methyl bisulfate were lower (Table 1, # 9 and 10), than with iodine and extensive poly-halogenated methanes typical of free-radical reactions were observed. This marked difference in reactivity between chlorine, bromine and iodine is consistent with the proposed involvement of iodo cations such as I2+ as it has been reported that the related cations of bromine and chlorine are not stable in oleum.9
The unusually high reaction efficiency and product selectivity for methane conversion, along with the strong dependence on solvent acidity is very similar to the reported, high yield oxidation of methane in sulfuric acid solvent to methyl bisulfate catalyzed by the soft, stable, redox-active electrophile, Hg(II).2 Based on the proposal that Hg(II) catalyzes methane oxidation via CH activation by electrophilic substitution,2 it is possible that the poorly coordinated I2+H2S2O7−, in spite of its known radical character,9 is sufficiently electrophilic, soft and stable to also react with methane by a predominantly electrophilic substitution pathway as shown in Fig. 1, that does not involve the formation of free radicals. The proposed electrophilic substitution by I2+ shown in Fig. 1, is not without precedent. Similar electrophilic substitutions of CH bonds have been proposed for the reaction of alkanes with H+, O3+, NO2+, and other electrophiles.6,13 The electrophilic substitution of arenes by aryl iodo cations is also known.14 Consistent with the proposed functionalization step in Fig. 1 and the observation that no free methyl iodide is formed, we find that addition of methyl iodide to 2.5% oleum at 150 °C leads to the immediate and quantitative formation of methyl bisulfate and blue colored species due to I2+. Consistent with the observation that I2 does not catalyze H/D exchange between D2SO4/SO3 and CH4, no methane is produced in this reaction. The proposed oxidation step in Fig. 1 has been reported.9,11 Elemental sulfur, selenium and tellurium are also reported to generate cationic species on dissolution in oleum and we have found that these species also catalyze reaction between methane and oleum, but at much lower efficiencies as compared to iodine.
 | ||
| Fig. 1 Proposed electrophilic CH activation mechanism for the iodine catalyzed oxidation of methane in oleum. | ||
Notes and references
- P. Burger and R. G. Bergman, J. Am. Chem. Soc., 1993, 115, 10462 CrossRef CAS; M. W. Holtcamp, J. A. Labinger and J. E. Bercaw, J. Am. Chem. Soc., 1997, 119, 848 CrossRef CAS; L. Johansson, D. D. Wick and K. I. Goldberg, J. Am. Chem. Soc., 1997, 119, 10235 CrossRef CAS; O. B. Ryan and M. Tilset, J. Am. Chem. Soc., 1999, 121, 1974 CrossRef CAS; J. T. Golden, R. A. Andersenand and R. G. Bergman, J. Am. Chem. Soc., 2001, 123, 5837 CrossRef CAS.
- R. A. Periana, D. J. Taube, S. Gamble, H. Taube, T. Satoh and H. Fuji, Science, 1998, 280, 560 CrossRef CAS; R. A. Periana, D. J. Taube, E. R. Evitt, D. G. Loffler, P. R. Wentrcek, G. Voss and T. Masuda, Science, 1993, 259, 340 CAS.
- In a typical reaction, a 50 ml glass-lined, stirred, high-pressure reactor containing 5 mM iodine and 2.5 wt% sulfur trioxide dissolved in 15 ml of concentrated sulfuric acid was pressurized with methane to a final pressure of 500 psig. This reaction mixture was vigorously stirred and maintained at 195 °C for 2 h before analysis by NMR and HPLC. The reaction must be properly mixed to avoid mass-diffusion control with respect to CH4.
- We did not examine oleum concentrations >5 wt% SO3 as many species are catalysts for the methane oxidation under those conditions. A patent (N. J Bjerrum, C. Radhusvej, G. Xiao, L. Bauneporten, H. A. Hjuler, R. K. Dreyervej, WO 99/24383) claiming the use of many materials including iodine as catalysts for conversion of methane to methyl bisufate in 65 wt% oleum was reported after we carried out our initial work. No mechanistic work was reported on this patent.
- A. Sen, Acc. Chem. Res., 1998, 31, 550 CrossRef CAS and references therein.
- G. A. Olah, G. K. S. Prakash and J. Sommer, Superacids, Wiley-Interscience, New York, 1985 Search PubMed.
- The formation of low levels of CH2DOSO3H is consistent with the oxidation of the low levels of CH3D that is formed.
- A. M. Rosan, J. Chem. Soc., Chem. Commun., 1985, 7, 377 RSC.
- R. J. Gillespie and M. J. Morton, Quart. Rev., 1971, 25, 553 Search PubMed; T. A. O’Donnel, Super Acid and Acidic Melts as Inorganic Chemical Reaction Media, VCH Publishers Inc, New York, 1992, Chapter 4 Search PubMed and references therein.
- R. J. Gillespi and J. B. Senio, Inorg. Chem., 1964, 3, 972 CrossRef CAS.
- F. Aubke, H. A. Carter and S. P. Jones, Inorg. Chem., 1970, 9, 2485 CrossRef CAS; A. Bali and K. C. Malhotra, J. Inorg. Nucl. Chem., 1976, 38, 411 CrossRef CAS.
- The reaction of iodine radicals with methane is highly endothermic and not a viable pathway for catalytic methane oxidation: D. M. Golden and S. W. Benson, Chem. Rev., 1969, 69, 125 Search PubMed.
- Such reaction may involve inner-sphere proton-coupled electron transfer: A. A. Fokin, T. E. Shubina, P. A. Gunchenko, S. D. Isaev, A. G. Yurchenko and P. R. Schreiner, J. Am. Chem. Soc., 2002, 124, 10718 Search PubMed.
- P. J. Stang and V. V. Zhdankin, Chem. Rev., 1996, 96, 1123 CrossRef CAS; V. A. Grushin, Chem. Soc. Rev., 2000, 29, 315 RSC.
| This journal is © The Royal Society of Chemistry 2002 |
