Controlled growth of triblock polyelectrolyte brushes
Vicky L.
Osborne
,
Darren M.
Jones
and
Wilhelm T. S.
Huck
*
University of Cambridge, Department of Chemistry, Melville Laboratory for Polymer Synthesis, Cambridge, UK CB2 3RA. E-mail: wtsh2@cam.ac.uk
First published on 23rd July 2002
Abstract
We have achieved a significant breakthrough in the synthesis of polyelectrolyte brushes of controlled thickness and density, which has been demonstrated by the synthesis of triblock copolymer brushes composed of cationic, neutral, and anionic segments.
Polymer brushes have been widely used to tailor surface properties such as wettability and friction and there is an increasing interest of using diblock copolymer brushes for ‘smart’ or responsive surfaces.1 The properties of tethered polyelectrolyte (PEL) brushes on solid surfaces have attracted considerable theoretical2 and experimental interest.3 Such brushes have been prepared either via physisorption of block copolymers,4 or via chemisorption of end-functionalized polymers.5 In both cases, the brush thickness and grafting density is limited, because of the diffusion barrier that develops after the grafting of the first polymer chains to the surface.6 Ideally, the synthetic method to functionalize surfaces with polymer brushes, should allow full control over the thickness, density and composition of the polymer films. Surface-initiated polymerizations of polymer brushes (or grafting from method) have been very successful in this controlled growth and a variety of polymer brushes has been grown using different ‘living’ polymerization conditions.7 Previously, we exploited the rapid increase in rate of Atom Transfer Radical Polymerizations (ATRP)8 to grow brushes (of water-soluble and water-insoluble polymers) from surfaces in aqueous media without losing control over the reaction.9 Despite the clear advantage of surface-initiated polymerizations, there have been no reports of the controlled growth of PEL brushes. Poly(styrene) (PS) and poly(vinyl pyridine) (PVP) brushes (synthesized via free radical techniques) have been sulfonylated or quaternized to generate PEL brushes.10 However, even under quite harsh conditions, these reactions often result in incomplete post-functionalization, which results in inhomogeneous PEL brushes.11 Secondly, the free radical polymerization yields high polydispersities, which makes the verification of many theoretical models based on monodisperse polymer chains difficult.12 Thirdly, there is no control over the density of the brushes (i.e. the spacing between the polymer brushes) which is again of theoretical importance.13
In order to achieve maximum control over brush density, polydispersity, and composition, plus at the same time allowing the formation of block copolymers on the surface, a controlled polymerization is highly desirable. Hence, surface-initiated aqueous ATRP should provide an ideal route to well-defined, homogeneous polyelectrolyte brushes.
Scheme 1 outlines the procedure for the preparation of polymer brushes. First, we used microcontact printing (μCP)14 on gold to prepare patterned monolayers of an initiator for ATRP with a methyl-terminated self-assembled monolayers (SAM) as an inert background. Non-patterned monolayers were formed by placing clean gold surfaces in a solution containing initiator, ‘diluted’ with undecanethiol when mixed monolayers were required.15 Secondly, the initiator surfaces were placed in the polymerization bath containing monomer, catalyst, ligand and solvent. After a set reaction time, the substrates were removed from the bath. No polymerization occurred in solution and a quick wash with water and methanol, to rinse off the polymerization solution, yielded clean surfaces.
 | ||
| Scheme 1 Schematic outline of brush formation. | ||
We measured the thickness of polymer brushes on homogeneous surfaces using ellipsometry. As a second technique, we used AFM to measure the film thickness on patterned surfaces. We chose [2-(methacryloyloxy)ethyl]trimethylammonium chloride (METAC) as the monomer for cationic brushes. The charges in these brushes are present on each monomer unit regardless of the pH of the solution. Fig. 1(a) shows the increase in brush thickness as a function of polymerization time for PMETAC brushes grown from different initiator densities. The data points are average values from separate measurements of at least three different spots on two or more different samples. The AFM clearly shows the clean and homogenous growth of the brushes from patterned SAMs (Fig. 2). Surface-initiated polymerizations from dilute SAMs afford control over brush grafting density. We have grown PMETAC brushes from 10, 25 and 100% initiator SAMs and found that we can control the thickness depending on initiator concentration (Fig. 1(a)). The Mw of the polymer brushes is only dependent on the reaction time and not on the initiator concentration in the monolayer: hence brushes with different thickness, but grown for the same amount of time must be spaced further apart. Thicknesses were measured by ellipsometry, with a value of 1.5 for the refractive index (a change in the refractive index by 0.1 changes the thickness value by 1 nm, which is within the experimental error to which we can control our brush growth). Advancing contact angles, θAW, were found to be 48°. Grazing angle FT-IR spectra exhibited an absorbance peak at 1730 cm−1, characteristic of an ester carbonyl stretch.
![(a) Controlled growth of P(METAC) brushes. Lines have been added to guide the eye. Reaction conditions = [METAC]∶[CuCl]∶[Bipy]∶[CuCl2] = 100∶2∶5∶0.1 MeOH∶H2O 4∶1; room temperature. (b) Controlled growth of P(NaMA) brushes. Reaction conditions [NaMA]∶[CuBr]∶[Bipy]∶[CuBr2] = 100∶2∶5∶0.1 H2O; 60 °C.](/image/article/2002/CC/b204737c/b204737c-f1.gif) | ||
| Fig. 1 (a) Controlled growth of P(METAC) brushes. Lines have been added to guide the eye. Reaction conditions = [METAC]∶[CuCl]∶[Bipy]∶[CuCl2] = 100∶2∶5∶0.1 MeOH∶H2O 4∶1; room temperature. (b) Controlled growth of P(NaMA) brushes. Reaction conditions [NaMA]∶[CuBr]∶[Bipy]∶[CuBr2] = 100∶2∶5∶0.1 H2O; 60 °C. | ||
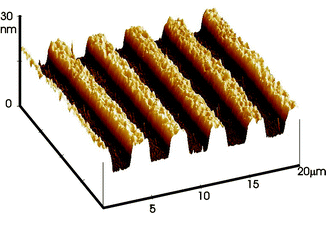 | ||
| Fig. 2 AFM image of a 30 nm PMETAC brush grown from a patterned surface. | ||
For the anionic brushes we used sodium methacrylate (NaMA). The aqueous ATRP of NaMA at 90 °C has been reported.16 but the polydispersities of the resulting polymers were slightly higher than expected for other monomers. The growth of the weak anionic electrolyte brushes was successful, but less controlled (Fig. 1(b)). We were not able to grow brushes at the high temperatures reported in the literature as the initiator SAMs would desorb from the surface. We therefore performed the polymerizations at 60 °C, which presumably leads to a slightly erratic growth. Both the anionic and cationic surfaces were hydrophilic, with advancing contact angles θAW = 56 and 48°, respectively, and receding contact angles close to 0° (the drops remained pinned). Grazing angle FT-IR showed a strong peak at 1720 cm−1, which we attributed to the carboxylic acid functionality. The absence of carboxylate absorptions indicates that rinsing the brush surfaces with water protonated the anion and removed the Na+ ions from the polymer film.
To demonstrate the controlled nature of the aqueous ATRP of charged monomers, we reinitiated the polycationic brushes in the presence of methyl methacrylate. This allowed the formation of polycationic–neutral block copolymers. Up to 7 nm thick PMMA blocks were grown on top of 5–20 nm thick PMETAC brushes. The advancing contact angles for the PMETAC-b-MMA brush changed from 48° after the first step, to 78° after the PMMA addition (lit. θAW = 71°). These diblocks were then used in the formation of triblock copolymer brushes consisting of cationic, neutral and anionic layers. The final block of this triblock copolymer brush was the methacrylic acid polymer. This reaction was again performed at 60 °C. The most obvious sign of successful reinitiation is the drop in the contact angles to θAW = 56°. Ellipsometry showed a clear increase in polymer film thickness. We used a bilayer model composed of a gold substrate, a PMETAC-b-MMA polymer layer and the unknown PNaMA layer. All refractive indices were taken as 1.5; the refractive index of PMMA of 1.49 will not significantly alter the thickness. The third block was measured to have an ellipsometric thickness of 6 nm. The IR spectrum showed a strong absorption at 1730 cm−1, with a small shoulder at both sides.
The electrostatic interactions of polyelectrolyte brushes determine their shape in solution.17 This ability to alter their conformation in response to external stimuli should lead to a richness of possible behaviours. Changes in salt concentrations or pH (for strong and weak electrolytes, respectively) should influence the brush height. Here, we report our initial attempts to study the behaviour of these PEL brushes in different environments. We measured the thickness of brushes as grown, and after placing the substrates in salt solutions for 1 h. We were not able to measure the change in situ; the measurements were performed on dried samples. This will distort the absolute values, but a general trend is easily observed. The thickness of PMETAC brushes grown from 100% initiator surfaces changed dramatically when exposed to 0.1 and 1 M KI solution (Table 1). Interestingly, the nature of the anion seems to have an influence on the degree of shrinkage of the brushes. Soaking in both 0.1 and 1 M NaCl solutions did not lead to any decrease in brush thickness, as measured by ellipsometry.
In conclusion, we have successfully synthesized a number of polycationic and polyanionic brushes using aqueous ATRP. Due to the controlled nature of our polymerization procedure, the PEL brushes could be used in the growth of complex di- and triblock copolymers from the surface. By varying the initiator density we were able to control the brush density and the thickness of the PEL layers. Surfaces functionalized with polyelectrolyte brushes can have important applications in e.g. biosensors and ‘smart’ coatings. The controlled synthesis of such films and the ability to pattern the surface, as demonstrated in this paper, will enable the development of PEL brushes in such applications, while at the same time it will lead to a further theoretical and fundamental understanding of the properties of these polymers.
We thank Prof. S. Armes for his helpful suggestions. This work was support by ICI, the Isaac Newton Trust, ICI-Vinamul (V. L. O.) and A. W. E. (DMJ).
Notes and references
- J.-W. Park and E. L. Thomas, J. Am. Chem. Soc., 2002, 124, 514 CrossRef CAS.
- P. Pincus, Macromolecules, 1991, 24, 2912 CrossRef CAS; S. Alexander, J. Phys., 1977, 38, 977 Search PubMed; P.-G. de Gennes, Macromolecules, 1980, 13, 1069 CrossRef CAS.
- J. Klein and E. Kumacheva, Science, 1995, 269, 816 CAS; J. Habicht, M. Schmidt, J. Rühe and D. Johansmann, Langmuir, 1999, 15, 2460 CrossRef CAS.
- C. Amiel, M. Sikka, J. W. Schneider, J. H. Tsao, M. Tirrell and J. W. Mays, Macromolecules, 1995, 28, 3125 CrossRef CAS.
- Y. Mir, P. Auroy and L. Auvray, Phys. Rev. Lett., 1995, 75, 2863 CrossRef CAS.
- Y. Tran, P. Auroy and L.-T. Lee, Macromolecules, 1999, 32, 8952 CrossRef CAS.
- O. Prucker and J. Rühe, Macromolecules, 1998, 31, 591; M. Husseman, E. E. Malmström, M. McNamara, M. Mate, D. Mecerreyes, D. G. Benoit, J. L. Hedrick, P. Mansky, E. Huang, T. P. Russell and C. J. Hawker, Macromolecules, 1999, 32, 1424 CrossRef.
- X. S. Wang, S. F. Jackson and S. P. Armes, Macromolecules, 2000, 33, 255 CrossRef CAS; X. S. Wang, R. A. Lascelles and S. P. Armes, Chem. Commun., 1999, 18, 1817 RSC; K. L. Robinson, M. A. Khan, M. V. de Paz Báñez, X. S. Wang and S. P. Armes, Macromolecules, 2001, 34, 3155 CrossRef CAS; X. S. Wang and S. P. Armes, Macromolecules, 2000, 33, 6640 CrossRef CAS.
- D. M. Jones and W. T. S. Huck, Adv. Mater., 2001, 13, 1256 CrossRef CAS.
- Y. Tran and P. Auroy, J. Am. Chem. Soc., 2001, 123, 3644 CrossRef CAS; M. Biesalski and J. Rühe, Macromolecules, 1999, 32, 2309 CrossRef CAS; M. Biesalski and J. Rühe, Langmuir, 2000, 16, 1943 CrossRef CAS.
- H.-J. Butt, M. Kappl, H. Meuller, R. Raiteri, W. Meyer and J. Rühe, Langmuir, 1999, 15, 2559 CrossRef CAS.
- S. T. Milner, T. A. Witten and M. E. Cates, Macromolecules, 1989, 22, 853 CrossRef CAS.
- A. Karim, S. K. Satija, J. F. Douglas, J. F. Ankner and L. J. Fetters, Phys. Rev. Lett., 1994, 73, 3407 CrossRef CAS.
- Y. Xia and G. M. Whitesides, Langmuir, 1997, 13, 2059 CrossRef CAS.
- D. M. Jones, A. A. Brown and W. T. S. Huck, Langmuir, 2002, 18, 1265 CrossRef CAS.
- E. J. Ashford, V. Naldi, R. O’Dell and S. P. Armes, Chem. Commun., 1999, 14, 1285 RSC.
- Y. Tran, P. Auroy, L.-T. Lee and M. Stamm, Phys.Rev. E., 1999, 60, 6984 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |
