Colour indicator for enantiomeric excess and assignment of the configuration of the major enantiomer of an amino acid ester
Richard A.
van Delden
and
Ben L.
Feringa
*
Department of Organic and Molecular Inorganic Chemistry, Stratingh Institute, University of Groningen, Nijenborgh 4, 9747 AG, Groningen, The Netherlands. E-mail: feringa@chem.rug.nl;; Fax: 31 50 3634296;; Tel: 31 50 3634278
First published on 11th January 2002
Abstract
A colour indicator for the full range of enantiomeric excess (−100% → 100% ee) is presented which is based on visual colour inspection of a liquid crystal doped with the analyte, i.e. the methyl ester of amino acid phenylglycine, providing the enantiomeric excess and allowing the assignment of the major enantiomer.
In the current pursuit for fast and easy assessment of enantioselectivity in asymmetric catalysis especially in combinatorial catalysis approaches, a simple and direct colour test for enantiomeric excess is highly desirable.1 Recently, we described a colour test for enantiomeric excess of simple organic molecules using doped liquid crystals.2 The method makes use of the unique optical properties of cholesteric phases obtained by doping achiral nematic liquid crystals with chiral dopants. Cholesteric liquid crystals are known to show a Bragg type incident angle (α) dependent reflection of light with a wavelength proportional to its refractive index (n) and pitch (p).3 The pitch is extremely sensitive towards chiral perturbations4 and inversely proportional to the concentration of chiral dopant (c), its intrinsic helical twisting power (β) and most important for the current application its enantiomeric excess (ee). These combined dependencies give an ee dependent wavelength of reflection following eqn. (1). With reflection wavelengths in the range of visible light this results in an ee dependent colour of the LC sample and as such offers the possibility of a colour indicator of chirality.
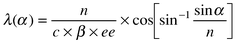 | (1) |
![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 50% can be visualized and assignment of the preponderant enantiomer is not possible. Here
we present a new version of the colour test as well as the application for an amino acid derivative. For the methyl ester of phenylglycine (1), colour inspection yields a full range ee determination as well as the possibility to assign the major stereoisomer.
50% can be visualized and assignment of the preponderant enantiomer is not possible. Here
we present a new version of the colour test as well as the application for an amino acid derivative. For the methyl ester of phenylglycine (1), colour inspection yields a full range ee determination as well as the possibility to assign the major stereoisomer.
Methyl phenyl glycine (1) itself has a negligible helical twisting power in E7 (the nematic liquid crystalline host used here) and as a consequence no cholesteric textures are observed upon doping with this amino acid ester. A simple imine-forming functionalisation reaction using p-methoxy biphenyl carbaldehyde (2) as a mesogenic unit, that is a unit structurally resembling the liquid crystalline host, affords imine 3 (Fig. 1). Due to the derivatisation, this compound has a high helical twisting power (β = + 16.0 μm−1 for (S)-3) and high compatibility with the liquid crystalline host E7 where upon doping up to 25 wt%, relatively stable liquid crystalline phases could still be observed.5†
 | ||
| Fig. 1 Functionalization of methyl phenylglycine with a mesogenic unit. | ||
As a consequence of its helical twisting power and its influence on the refractive index of the liquid crystalline sample a concentration of 18.5 wt% of enantiomerically pure dopant 3 is required to generate a violet coloured LC sample. The coloured phases are formed spontaneously by allowing a toluene solution of the appropriate amounts of dopant and liquid crystal to evaporate on a pre-aligned polyimide covered glass surface. Besides direct visual colour inspection the wavelength of reflection was measured spectroscopically to be 337 nm where the incident angle of the light was 45°. The average refractive index of the doped LC material is distinctly lower than for undoped E7 allowing colour detection at lower concentrations than expected. Like in the original colour indicator of chirality, changing the ee gradually to 50 wt% would result in a red shift of the reflection wavelength that could then be monitored spectroscopically as well as visually. Therefore, again our LC based method functions as a colour indicator for ee.
Since no chiral auxiliaries are used the method as described above does not discriminate between the two enantiomers of an analyte, although it should be noted that employing chiral spectroscopic techniques discrimination is possible. Furthermore, it allows accurate visual screening for enantiomeric excesses above 50%. However, upon decreasing the concentration of dopant the visualisable range is shifted to lower ee’s. Of course for initial screening in asymmetric catalysis, information on the most abundant enantiomer is not essential and neither are catalysts or conditions that show low enantioselectivity (that is ee’s lower than 50% in the product). For laboratory purposes however this information is often required and therefore we have adjusted the method to be able to obtain this information using enantiomerically pure imine 3 itself as a chiral auxiliary. As indicated above, upon doping only with analyte 3 of unknown enantiopurity a colour indicator for ee’s above 50% is obtained. Instead of doping with 18.5 wt% of analyte, a mixture of analyte and enantiomerically pure (S)-3 (chiral auxiliary) (ratio 1∶3), is now used as dopant. The composition of the LC sample is then 4.6 wt% of analyte and 13.9 wt% of chiral auxiliary. First, E7 was doped only with the chiral auxiliary in the appropriate amount of 13.9 wt%. This LC sample without analyte showed a yellowish colour and a corresponding wavelength of reflection of 534 nm when measured at a 45° angle. This wavelength is in the centre of the visible light spectrum. Next, samples were prepared by adding analyte 3 with ee’s ranging from 100% (S)-3 to 100% (R)-3 in steps of 20% ee to obtain the desired 4.6 wt% of analyte and 18.5 wt% of total dopant. The reflection wavelengths of these co-doped samples were measured (Fig. 2).
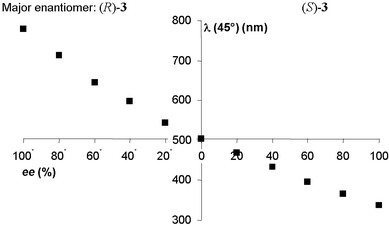 | ||
| Fig. 2 Wavelength of reflection as a function of ee of dopant. | ||
When the analyte is enantiomerically pure (S)-3 imine, the violet coloured sample with a reflection wavelength of 337 nm is obtained. Upon decreasing ee of the analyte the wavelength of reflection is shifted to the red as seen from Fig. 2. In the case of racemic analyte the obtained doped LC sample has a net dopant ee of 75%, leading to a yellowish colour similar to the colour of the starting mixture of E7 with 13.9 wt% of chiral auxiliary with a reflection wavelength of 504 nm. Theoretically the pitch of both samples should be identical, the difference of 30 nm is due to the decrease of average refractive index of the LC sample upon increasing dopant concentration, in accordance with the significantly lowered refractive index observed for all doped samples compared to pure E7.
For all samples where the analyte is of the same configuration as the chiral auxiliary, in this case the (S)-configuration, the wavelength of reflection is shifted towards the violet end of the spectrum compared to that of the racemic analyte. When samples of analyte with different ee but now with the other stereoisomer (R)-3 being the most abundant, the wavelength of reflection is further red shifted to a measured values of 779 nm for enantiomerically pure (R)-analyte. Using the ee dependent reflection wavelength curve depicted in Fig. 2 as a calibration curve the unknown ee of an analyte (in the present case the derivative of phenylglycine methyl ester) can be determined very accurately. The exact accuracy is wavelength and thus ee dependent but assuming that the effect of the refractive index is negligible in a short wavelength range the minimum and maximum accuracy can be calculated from the above equation. The minimum wavelength shift per percentage ee change (that is the wavelength shift going from 100% to 99% ee of (S)-analyte) is 0.8 nm. The maximum shift (going from 99% to 100% of (R)-analyte) is 7.7 nm.
Although reflection wavelength measurements offer an elegant method for accurate ee determination, the real advantage of this approach lies in the fact that all these wavelength shifts can also be instantaneously observed by the human eye as a change in colour. Direct visualisation is the fastest thinkable screening technique. For the analyte 3 the observed colour together with the reflection wavelengths measured are summarised in Table 1. Differences in measured and visually observed colours can be explained by the angle dependency of the reflection wavelength, where measurements are performed at a 45° angle and visualisation is done perpendicular.
| ee analyte (%) | Net ee dopant (%) | λ(45°) (nm) | Colour |
|---|---|---|---|
| a Ee where the (R)-enantiomer is most abundant. | |||
| 100 | 100 | 337 | Violet |
| 80 | 95 | 366 | Violet–blue |
| 60 | 90 | 396 | Blue |
| 40 | 85 | 434 | Blue–green |
| 20 | 80 | 468 | Green |
| 0 | 75 | 504 | Yellow |
| 20a | 70 | 544 | Yellow–orange |
| 40a | 65 | 598 | Orange |
| 60a | 60 | 644 | Red |
| 80a | 55 | 714 | Deep red |
| 100a | 54 | 779 | Red glow |
In conclusion, a colour indicator of ee for the methyl ester of phenyl glycine, based on functionalisation with a mesogenic unit and doping in an LC phase, is presented. By using the enantiomerically pure functionalisation product as a chiral auxiliary dopant the full range of enantiopurity can be visually detected by monitoring the colour of the sample. Discrimination between the two enantiomers can be done readily by monitoring either a blue or a red shift in colour compared to racemic analyte. A blue shift in this case corresponds to an analyte where the most abundant enantiomer has the same configuration as the chiral auxiliary. As such the method presented here offers a major extension compared to the originally reported colour test; the same principles were shown to hold for a biologically significant amino acid derivative, whereas in addition full range ee determination as well as assignment of the major enantiomer by a simple colour test is shown for the first time.
Notes and references
- M. T. Reetz, Angew. Chem., Int. Ed., 2001, 40, 284 CrossRef CAS.
- R. A. van Delden and B. L. Feringa, Angew. Chem., Int. Ed., 2001, 49, 3198 CrossRef CAS.
- D. Dunmae and K. Toniyama, Handbook of Liquid Crystals, Vol 1: Fundamentals, Wiley, Weinheim, 1998, pp. 215–239. Search PubMed.
- G. Solladié and R. G. Zimmermann, Angew. Chem., Int. Ed., 1984, 23, 348 CrossRef.
- Pitches, and indirectly the helical twisting power, are determined using a Grandjean Cano technique; G. Heppke and F. Oestreicher, Mol. Cryst. Liq. Cryst., 1977, 41, 245 Search PubMed The sign was determined by a contact method; N. Isaert, B. Soulestin and J. Malthète, Mol. Cryst. Liq. Cryst., 1976, 37, 321 Search PubMed.
Footnote |
| † Aligned cholesteric phases were obtained in the following way: a glass surface (typically 6.25 cm2) was spin-coated with commercially available polyimide AL1051 (JSR, Japan) and the coated surfaces were allowed to harden at 170 °C in vacuo for 3 h. The surface was then linearly rubbed with a velvet cloth to induce parallel aligned LC phases. The LC material E7 (commercially available from Merck, Germany) doped with the appropriate amount of dopant material was dissolved in toluene (approx. 5 mg ml−1) and poured onto the surface. Suitable aligned LC films were obtained after evaporation of the toluene at rt. For the LC samples doped with 18.5 wt% 3, n is approximately 1.3 compared to 1.6 for pure E7; both values are wavelength dependent and show an increase upon increasing wavelength. |
| This journal is © The Royal Society of Chemistry 2002 |
