Novel water clusters in the crystalline state: structures of a symmetrical, cyclic hexamer and an ‘opened-cube’ octamer
Robert J.
Doedens
*a,
Elizabeth
Yohannes
b and
M. Ishaque
Khan
b
aDepartment of Chemistry, University of California, Irvine, CA 92697, USA. E-mail: rdoedens@uci.edu
bDepartment of Biological, Chemical, and Physical Sciences, Illinois Institute of Technology, Chicago, IL 60616, USA
First published on 2nd January 2002
Abstract
Six- and eight-membered hydrogen-bonded water clusters of novel structure types have been found in crystalline hydrates.
Small water clusters, [(H2O)n, n = 2–10], have been a topic of considerable recent interest.1,2 Studies of water clusters can yield insight into the properties of water in various environments and clusters have played a role in theoretical approaches to understanding the properties of bulk water.3 The most stable conformations for water clusters of various degrees of aggregation have been predicted on the basis of ab initio electronic structure calculations.4 A number of clusters, including hexamers and octamers, have been characterized spectroscopically in the gas phase,5–7 in molecular beams8 and in liquid helium droplets.9 Clusters with n = 6, 8 and 10 have been found in crystalline hydrates.10–14
For n = 6, the calculated minimum-energy structure is a three-dimensional cage,4 which is consistent with experimental data for isolated clusters.5 Cyclic hexamers, predicted to be only slightly higher in energy, have been found in liquid helium droplets9 and in three solid-state systems. In chiral crystals of tris-(2′-methylbenzamidazol-1′-yl)methane, the (H2O)6 rings have an envelope conformation and are linked into chains by a seventh water molecule. The racemic form of the same compound contains isolated six-membered rings with a chair conformation.10 Water hexamers, linked into one-dimensional tapes, have been found to occupy the channels in crystals of a π-stacked benzonapthyridine derivative.11
Here, we describe a symmetrical, cyclic hexameric water cluster observed in the crystalline framework material Li6[Ni3V18O42(H2O)12(SO4)]·24H2O 1,15 derived from the cage-like polyoxovanadate cluster {V18O42(SO4)}. Compound 1 is isomorphous with our previously reported Fe and Co analogs,16 but forms higher quality crystals that permit observation of more complete details of the water cluster.17 The body-centered cubic unit cell contains eight equivalent hydrogen-bonded (H2O)6 clusters with a chair configuration and crystallographic ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry. Fig. 1 is an edge-on view of the unit cell in which four of the water clusters are visible and Fig. 2
shows the structure of a single hexameric water cluster. The hydrogen atoms within the {(H2O)6} ring display a twofold disorder and the axial hydrogen atom is hydrogen-bonded to one of the terminal oxygen atoms of the {V18O42(SO4)} cage.
m symmetry. Fig. 1 is an edge-on view of the unit cell in which four of the water clusters are visible and Fig. 2
shows the structure of a single hexameric water cluster. The hydrogen atoms within the {(H2O)6} ring display a twofold disorder and the axial hydrogen atom is hydrogen-bonded to one of the terminal oxygen atoms of the {V18O42(SO4)} cage.
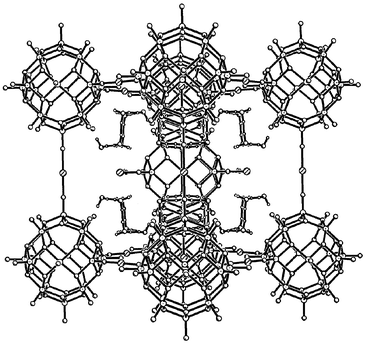 | ||
| Fig. 1 An edge-on view of the body-centered unit cell of 1, showing the {V18O42(SO4)} cages, the Ni2+ ions linking them, and the hexameric water clusters. For clarity, the encapsulated sulfate ions, the water molecules bound to the nickel ion and the Li+ ions have been omitted. Four of the eight water clusters are clearly visible; the others are obscured by the cages. | ||
 | ||
| Fig. 2 The cyclic water cluster in 1, showing the disorder of the equatorial hydrogen atoms. Each equatorial hydrogen atom is hydrogen bonded to the adjacent oxygen atom. | ||
The chair configuration and the disordered hydrogen atoms of the (H2O)6 ring are also found in hexagonal ice (ice Ih) as well as in the metastable cubic ice Ic.18 The intra-ring O⋯O distance of 2.759 Å in deuterated ice Ih19 is close to the value of 2.769 Å that we find. However, the torsion angle of the chair in the present case (77.7°) is considerably larger than the corresponding value of 60.2° in ice. Among crystallographically characterized six-membered clusters, ours is the first example to display the full symmetry and the hydrogen atom disorder of the hexagonal building block of ice.
For n = 8, theory predicts two closely related isomers of nearly identical energy with S4 and D2d symmetries.4 Each of these isomers has oxygen atoms at the corners of a cube with hydrogen bonds along each edge; they differ only in the details of the hydrogen bonding. Evidence for the presence of both of these isomers has been found in gas-phase C6H6(H2O)8 clusters7 and in molecular beams.8 An octameric cluster with a cubic arrangement of oxygen atoms has also been reported in a solid-state hydrate, but in this case the hydrogen atoms were not located.12 Very recently, a cyclic (H2O)8 cluster that closely resembles a portion of the ice Ic structure has been found in an organic supramolecular complex.14
We have now found a new type of octameric cluster in crystalline [V(phen)2SO4]2O(H2O)4 (phen = 1,10-phenanthroline) 2, (Fig. 3).17,20 As shown in Fig. 4, this compound adopts a structure in which alternating layers of the oxygen-bridged complex and water clusters are stacked perpendicular to the a-axis. The water molecules, which are hydrogen-bonded to oxygen atoms of metal-coordinated sulfate groups, form centrosymmetric octameric clusters.
 | ||
| Fig. 3 A view of the oxygen-bridged dimer of 2. The two halves of the molecule are related by a twofold symmetry axis. | ||
 | ||
| Fig. 4 The crystal packing of 2, viewed down the c-axis and showing the alternating layers of O-bridged dimers and water clusters. For clarity, the carbon atoms of the 1,10-phenanthroline ligands have been omitted. | ||
A view of a single (H2O)8 cluster and its immediate environment in 2 is shown in Fig. 5. The cluster can be considered as derived from a cubic arrangement by the opening of two edges, resulting in the folding of two opposite faces into a ‘butterfly’ shape with a folding angle of 29.5°. The hydrogen-bonded O⋯O distances within the octamer range from 2.76 to 2.91 Å, while the oxygen atoms are separated by 3.92 Å along the opened edge. Each of the four independent water molecules donates one hydrogen atom to a hydrogen bond within the four-membered folded face. Three of these water molecules use their second hydrogen to form a hydrogen bond to a sulfate oxygen atom and the fourth forms a hydrogen bond to a water oxygen atom from the opposite face. One water oxygen atom serves as an acceptor for two hydrogen bonds and the others are single acceptors. A similar ‘opened-cube’ configuration with a different arrangement of hydrogen bonds is found at the core of the cage structure observed for the (H2O)10 cluster in molecular beams.8
 | ||
| Fig. 5 A centrosymmetric, octameric water cluster and its immediate environment as found in 2. All hydrogen bonds are shown. | ||
These results further illustrate the structural diversity of water clusters and the sensitive dependence of their structures upon the details of their environment.
M. I. K. acknowledges the funding support from the American Chemical Society’s Petroleum Research Fund (ACS-PRF# 35591-AC5).
Notes and references
- K. Liu, J. D. Cruzan and R. J. Saykally, Science, 1996, 271, 933–933 CrossRef CAS.
- J. M. Ugalde, I. Alkorta and J. Elguero, Angew. Chem., Int. Ed., 2000, 39, 717–721 CrossRef CAS.
- F. Weinhold, J. Chem. Phys., 1998, 109, 367–372 CrossRef CAS; F. Weinhold, J. Chem. Phys., 1998, 109, 373–384 CrossRef CAS.
- J. K. Gregory and D. C. Clary, J. Phys. Chem., 1996, 100, 18014–18022 CrossRef CAS.
- K. Liu, M. G. Brown and R. J. Saykally, J. Phys. Chem. A, 1997, 101, 8995–9010 CrossRef CAS.
- R. N. Pribble and T. S. Zwier, Science, 1994, 265, 75–79 CrossRef CAS.
- C. J. Gruenloh, J. R. Carney, C. A. Arrington, T. S. Zwier, S. Y. Fredericks and K. D. Jordan, Science, 1997, 276, 1678–1681 CrossRef CAS.
- U. Buck, I. Ettischer, M. Melzer, V. Buch and J. Sadlej, Phys. Rev. Lett., 1998, 80, 2578–2581 CrossRef CAS.
- K. Nauta and R. E. Miller, Science, 2000, 287, 293–295 CrossRef CAS.
- C. Foces-Foces, F. H. Cano, M. Martinez-Ripoll, R. Faure, C. Roussel, R. M. Claramunt, C. Lopez and D. Sanz, Tetrahedron: Asymmetry, 1990, 1, 65–86 CrossRef CAS.
- R. Custelcean, C. Afloroaei, M. Vlassa and M. Polverejan, Angew. Chem., Int. Ed., 2000, 39, 3094–3096 CrossRef CAS.
- W. B. Blanton, S. W. Gordon-Wylie, G. R. Clark, K. D. Jordan, J. T. Wood, U. Geiser and T. J. Collins, J. Am. Chem. Soc., 1999, 121, 3551–3552 CrossRef CAS.
- L. J. Barbour, G. W. Orr and J. L. Atwood, Nature, 1998, 393, 671–673 CrossRef; L. J. Barbour, G. W. Orr and J. L. Atwood, Chem. Commun., 2000, 859–860 RSC.
- J. L. Atwood, L. J. Barbour, T. J. Ness, C. L. Raston and P. L. Raston, J. Am. Chem. Soc., 2001, 123, 7192–7193 CrossRef CAS.
- Synthesis of 1. The reaction of hydrazinium sulfate (2.5 mmol) with a hot aqueous solution (13 mL) of lithium vanadate (5 mmol) prepared by the reaction of the stoichiometric amount of V2O5 (2.5 mmol) with LiOH.H2O (5 mmol) in water at 84–86 °C gave a dark colored solution. After diluting the resulting solution to 25 mL with deionized water, it was treated with NiSO4·6H2O (1.25 mmol) and the reaction mixture was heated at 84–86 °C for 2 h. The resulting dark solution yielded bluish-black crystals of 1. A full report on the synthesis, characterization, and properties of 1 will be published elsewhere..
- M. I. Khan, E. Yohannes and R. J. Doedens, Angew. Chem., Int. Ed., 1999, 38, 1292–1294 CrossRef.
-
Crystal data: for 1: H72Li6Ni3O82SV18, M = 2551.33, cubic, space group Im
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 229), a = 15.4344(4) Å, U = 3676.8(2) Å3, T = 163 K, Z = 2, μ(Mo-Kα) = 3.077 mm−1, 12057 reflections measured, 488 unique (Rint
= 0.021) which were used in all calculations. The final R1 was 0.0353 (all data). The SO42− group is disordered and encapsulated within the polyoxovanadate cage. Some of the H2O molecules bound to Ni2+ and some of the Li+ ions are disordered. For 2: C24H24N4O8.5SV, M = 587.47, monoclinic, space group C2/c (no. 15), a = 21.2997(12), b = 14.1986(8), c = 16.3825(9) Å, β = 98.0460(10)°, U = 4905.7(5) Å3, T = 158 K, Z = 8, μ(Mo-Kα) = 0.551 mm−1, 25833 reflections measured, 5962 unique (Rint = 0.0382) which were used in all calculations. The final R1 value (all data) was 0.0548. A full report on the preparation, characterization and properties of the compound 2 will be included in
a future publication. CCDC reference numbers 171785 and 171786. See http://www.rsc.org/suppdata/cc/b1/b108866a/ for crystallographic data in CIF or other electronic format..
m (no. 229), a = 15.4344(4) Å, U = 3676.8(2) Å3, T = 163 K, Z = 2, μ(Mo-Kα) = 3.077 mm−1, 12057 reflections measured, 488 unique (Rint
= 0.021) which were used in all calculations. The final R1 was 0.0353 (all data). The SO42− group is disordered and encapsulated within the polyoxovanadate cage. Some of the H2O molecules bound to Ni2+ and some of the Li+ ions are disordered. For 2: C24H24N4O8.5SV, M = 587.47, monoclinic, space group C2/c (no. 15), a = 21.2997(12), b = 14.1986(8), c = 16.3825(9) Å, β = 98.0460(10)°, U = 4905.7(5) Å3, T = 158 K, Z = 8, μ(Mo-Kα) = 0.551 mm−1, 25833 reflections measured, 5962 unique (Rint = 0.0382) which were used in all calculations. The final R1 value (all data) was 0.0548. A full report on the preparation, characterization and properties of the compound 2 will be included in
a future publication. CCDC reference numbers 171785 and 171786. See http://www.rsc.org/suppdata/cc/b1/b108866a/ for crystallographic data in CIF or other electronic format.. - D. Eisenberg and W. Kauzmann, The Structure and Properties of Water, Oxford University Press, Oxford, 1969. Search PubMed.
- S. W. Peterson and H. A. Levy, Acta Crystallogr., 1957, 10, 70–76 CrossRef CAS.
- The hydrothermal reaction of vanadium pentoxide, 1,10-phenanthroline, hydrazinium sulfate, zinc sulfate heptahydrate and water in the molar ratio 1∶2∶1∶1.5∶444 for 120 h at 160 °C gave a deep colored liquid that was allowed to stay at room temperature for 24 h to yield purple crystals of 2 in moderate yield..
| This journal is © The Royal Society of Chemistry 2002 |
