Single phase preparation of monodispersed silver nanoclusters using a unique electron transfer and cluster stabilising agent, triethylamine
Nirmalya K.
Chaki
a,
Surendra G.
Sudrik
*b,
Harikrishan R.
Sonawane
b and
K.
Vijayamohanan
*a
aPhysical and Materials Chemistry Division, National Chemical Laboratory, Dr. Homi Bhaba Road, Pune, 411008, India. E-mail: viji@ems.ncl.res.in
bOrganic Chemistry Division (Technology), National Chemical Laboratory, Dr. Homi Bhaba Road, Pune, 411008, India
First published on 11th December 2001
Abstract
A simple and reproducible single phase preparation of 2.5 nm silver nanoclusters is described using silver benzoate along with triethylamine (TEA) and dodecanethiol (DDT); these spontaneously self-assemble to a two-dimensional array whereas in the absence of thiol only polydispersed nanoclusters are obtained.
The physico-chemical properties of metal nanoclusters are dependent on the number of atoms present in the clusters (size and shape control) and these properties are used in several fundamental and application purposes in diverse fields such as colloidal chemistry, physics, inorganic chemistry, physical chemistry, materials science and biological sciences.1–4 One of the principal objectives of various synthetic strategies of these materials is to achieve precise control over their size, shape and dispersion.5,6 The widely used Brust method consists of a biphasic system where a reducing agent such as NaBH4 along with an appropriate capping agent in an organic phase is used to generate metal nanoclusters.7 Since NaBH4 can cause a sudden pH change of the aqueous phase subsequent to the generation of cluster nuclei, its adverse effect on the growth rate of the clusters can be avoided by the use of a mild reducing agent. Furthermore, the high diffusion coefficient of silver ions in water (dielectric constant, ε ≈ 80) can accelerate the growth of the particle size, and hence the use of low dielectric constant solvents is important to restrict the growth.8 Consequently, we carried out the synthesis in a low dielectric medium (ε of toluene is 2.38) using a soluble silver salt of a carboxylic acid and a tertiary amine as an electron transfer agent as well as cluster stabilising agent. TEA was selected on the basis of its unique properties as a reducing agent in photochemical reactions9 and also because the amine group is known to stabilise nanoclusters.10 In addition, TEA is also miscible with most organic solvents and hence a drastic change in pH is not observed during the synthesis. Herein, we report the simultaneous use of a tertiary amine and DDT as reducing and capping agents, respectively, for the first time, and stable monodispersed silver nanoclusters can be prepared from silver benzoate as a homogeneous solution in toluene. Comparison of these DDT capped silver nanoclusters with those prepared using TEA alone using an otherwise identical synthetic protocol gives polydispersed solutions stable only for a few days. The formation of Ag nanoclusters has been confirmed by the characteristic silver plasmon band in UV-vis absorption spectroscopy11,12 and also by cyclic voltammetry.† The monodispersity and the remarkable ability to form 2D arrays are the unique features of these DDT capped Ag nanoparticles13 as revealed by transmittance electron microscopy (TEM)‡ images, recorded by drop casting the Ag-cluster solution in toluene at room temperature on a carbon coated copper grid.
The nanoclusters were synthesised in toluene (AR grade), using TEA as an electron transfer agent, and silver benzoate as the metal ion source. Briefly, 2 mM silver benzoate was dissolved in toluene by vigorous stirring followed by addition of DDT such that the metal ion to DDT concentration ratio was 1∶1. After 30 min vigorous stirring, 100 mM TEA (in toluene) was added dropwise and the reaction mixture stirred vigorously overnight. The resulting colourless solution was shaken with 10 mM NaHCO3 solution in order to remove excess benzoic acid and then the resulting toluene solution was dried under N2 atmosphere. Nanoclusters without DDT were synthesised using the same synthetic protocol. In this case the resulting solution was yellow.
Formation of silver nanoclusters was ascertained from the characteristic plasmon band in their optical spectra. Fig.1 shows the superimposed UV-vis spectra of both types of silver nanoclusters (with/without DDT) in toluene taken under identical conditions. Silver nanoclusters prepared using TEA alone (a) display a broad and unsymmetric silver surface plasmon band at 434 nm with a full width at half minimum (FWHM) of ca. 96 nm. More significantly, this plasmon band shows a slight red shift with time (ca. 4 nm shift after 1 week) concomitant with a broadening, which indicates an increase of the cluster size. In sharp contrast, the simultaneous use of DDT and TEA (b) gives a colourless homogeneous solution of silver nanoclusters exhibiting an intense and symmetric plasmon band at 348 nm with a FWHM of about ca. 68 nm. In addition, these plasmon characteristics were found to be invariant for two months.
 | ||
| Fig. 1 Uv-vis absorbance spectra of (a) Ag nanoclusters prepared using only TEA and (b) Ag nanoclusters prepared using TEA along with DDT. | ||
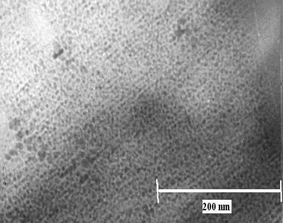
Fig. 2(a) shows a typical TEM image of clusters prepared with TEA alone where a polydispersed size distribution (1.4 ± 0.2 to 28.6 ± 0.2 nm) with an average particle size of 4.5 ± 0.2 nm can be seen. In comparison, the TEM image of clusters prepared using DDT and TEA (Fig. 2(b)) simultaneously reveals the presence of a highly monodispersed distribution with an average particle size of 2.5 ±0.3 nm (corresponding histograms of Fig.2 (a) and (b) shown as insets). A high resolution TEM (Fig. 3) interestingly illustrates array like features for these clusters with an inter-cluster spacing of ca. 2.5 nm. This along with the facetting is attributed to the formation of an ordered superstructure, perhaps, due to the hydrophobic interactions of the long alkyl chains.14,15
 | ||
| Fig. 2 TEM images of Ag nanoclusters prepared (a) only with TEA (b) by simultaneous use of TEA along with DDT (insets show the corresponding histograms of the size distribution of the clusters). | ||
Nevertheless, the presence of these alkyl chains does not obstruct electron transfer behaviour from the cores as indicated by the superimposed cyclic voltamograms of both types of silver nanoclusters (Fig. 4). For example, cyclic voltammogram (b) of Ag clusters (prepared with the simultaneous use of TEA and DDT) in 0.1 M LiClO4 dissolved in aqueous acetonitrile solution shows a redox couple centred at E1/2 = 128.5 mV vs. Ag/AgCl with a peak separation (ΔEp) of 229 mV. The sharp and symmetrical anodic peak (Epa) at 243 mV has a FWHM of 65 mV, signifying one electron oxidation. In addition, the broad cathodic peak (Epc = 14 mV; FWHM = 135 mV) can be deconvoluted to two small peaks, indicating that different electrochemical reduction stages may be present. In sharp contrast, the voltammogram of (a) Ag nanoclusters prepared with TEA alone under identical conditions exhibits an oxidation peak (Epa) at 214 mV with a FWHM of 65 mV which signifies that these clusters prefer to exist in oxidised form in solution. Thus the agglomeration observed in the Uv-vis spectra in Fig. 1 is in good agreement with the voltammetric results. These unique differences in the redox features of both type of silver nanoclusters can be effectively utilised in suitable electrocatalytic synthesis with Agn/Agn+ mediators.
 | ||
| Fig. 4 Superimposed cyclic voltammograms of silver nanoclusters prepared without (a) and with DDT (b). | ||
In conclusion, we have successfully demonstrated the formation of highly monodispersed silver nanoclusters in an anhydrous organic medium with low dielectric constant by use of a homogeneous silver salt (silver benzoate) solution and triethylamine as electron transfer agent. The simultaneous use of DDT and TEA endows several advantages such as prolonged stability, fast electron-transfer and narrow size distribution compared to other methods of silver nanocluster synthesis.
N. K. C. and S. G. S. acknowledge the Council of Scientific and Industrial Research (CSIR) for JRF and RA fellowships, respectively.
Notes and references
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 4212 and references therein Search PubMed.
- S.-H. Kim, G. Medeiros-Ribeiro, D. A. A. Ohlberg, R. Stanley Williams and J. R. Heath, J. Phys. Chem. B, 1999, 103, 10341 CrossRef CAS.
- G. Schmid and L. F. Chi, Adv. Mater., 1998, 10, 515 CrossRef CAS.
- C. A. Mirkin, Inorg. Chem., 2000, 39, 2258 CrossRef CAS.
- M. M. Alvarez, J. T. Khoury, T. G. Schaaff, M. N. Shafigullin, I. Vezmar and R. L. Whetten, J. Phys. Chem. B, 1997, 101, 3706 CrossRef CAS.
- T. S. Ahmadi, Z. L. Wang, T. C. Green, A. Henglein and M. A. El-Sayed, Science, 1996, 272, 1924 CrossRef CAS.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801 RSC.
- K. J. Ziegler, R. C. Doty, K. P. Johnston and Brain A. Korgel, J. Am. Chem. Soc., 2001, 123, 7797 CrossRef CAS.
- S. G. Cohen, A. Parola and G. H. Parsons, Chem. Rev., 1973, 73, 141 CrossRef CAS.
- M. Green and P. O’Brien, Chem. Commun., 2000, 183 RSC.
- A. Henglein, J. Phys. Chem., 1993, 97, 5457 CrossRef CAS.
- J. A. Creighton and D. G. Eadon, J. Chem. Soc., Faraday Trans., 1991, 87, 3881 RSC.
- K. Abe, T. Hamada, Y. Yoshida, N. Tanigaki, H. Takiguchi, H. Nagasawa, M. Nakamoto, T. Yamaguchi and K. Yase, Thin Solid Film, 1998, 329, 524 and references therein Search PubMed.
- S. He, J. Yao, P. Jiang, D. Shi, H. Zhang, S. Xie, S. Pang and H. Gao, Langmuir, 2001, 17, 1571 CrossRef CAS.
- A. Harfenist, Z. L. Wang, M. M. Alvarez, I. Vezmar and R. L. Whetten, J. Phys. Chem., 1996, 100, 13904 CrossRef CAS.
Footnotes |
| † Cyclic voltammograms were recorded on an EG & G pontentiostat using Pt wire as working electrode along with Pt foil as counter electrode and a Ag/AgCl reference electrode in an aqueous acetonitrile solution of LiClO4 (0.1 M); (CH3CN–H2O, 100∶10 v/v, 10 ml) at a scan rate of 4 mV s−1. |
| ‡ TEM images were recorded on a JEOL model 1200EX instrument operated at an accelerating voltage of 120 kV, by drop casting of an Ag-cluster solution in toluene at room temperature on a carbon coated copper grid. |
| This journal is © The Royal Society of Chemistry 2002 |