Nanomedicine embraces cancer radio-immunotherapy: mechanism, design, recent advances, and clinical translation
Haonan
Li†
 a,
Qiang
Luo†
a,
Hu
Zhang
a,
Qiang
Luo†
a,
Hu
Zhang
 c,
Xuelei
Ma
a,
Zhongwei
Gu
c,
Xuelei
Ma
a,
Zhongwei
Gu
 a,
Qiyong
Gong
*ab and
Kui
Luo
a,
Qiyong
Gong
*ab and
Kui
Luo
 *ab
*ab
aDepartment of Radiology, Department of Biotherapy, Huaxi MR Research Center (HMRRC), Cancer Center, Frontiers Science Center for Disease-Related Molecular Network, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, No. 37 Guoxue Alley, Chengdu 610041, China. E-mail: qiyonggong@hmrrc.org.cn; luokui@scu.edu.cn
bFunctional and Molecular Imaging Key Laboratory of Sichuan Province and Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, China
cAmgen Bioprocessing Centre, Keck Graduate Institute, Claremont, CA 91711, USA
First published on 25th November 2022
Abstract
Cancer radio-immunotherapy, integrating external/internal radiation therapy with immuno-oncology treatments, emerges in the current management of cancer. A growing number of pre-clinical studies and clinical trials have recently validated the synergistic antitumor effect of radio-immunotherapy, far beyond the “abscopal effect”, but it suffers from a low response rate and toxicity issues. To this end, nanomedicines with an optimized design have been introduced to improve cancer radio-immunotherapy. Specifically, these nanomedicines are elegantly prepared by incorporating tumor antigens, immuno- or radio-regulators, or biomarker-specific imaging agents into the corresponding optimized nanoformulations. Moreover, they contribute to inducing various biological effects, such as generating in situ vaccination, promoting immunogenic cell death, overcoming radiation resistance, reversing immunosuppression, as well as pre-stratifying patients and assessing therapeutic response or therapy-induced toxicity. Overall, this review aims to provide a comprehensive landscape of nanomedicine-assisted radio-immunotherapy. The underlying working principles and the corresponding design strategies for these nanomedicines are elaborated by following the concept of “from bench to clinic”. Their state-of-the-art applications, concerns over their clinical translation, along with perspectives are covered.
1. Introduction
In recent years, cancer immunotherapy, harnessing the innate or adaptive host immune systems to combat cancer, has enjoyed a flourishing growth rate. Generally, it is divided into two major antineoplastic approaches: active cancer vaccines for prophylactic or therapeutic use and passive immuno-oncology treatments.1 Passive treatments include administration of immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1),2 programmed death ligand-1 (PD-L1) or cytotoxicity T-lymphocyte-associated protein 4 (CTLA-4),3,4 injection of chimeric antigen receptor T-cell therapy (CAR T),5 bispecific antibodies (bsAbs, e.g., blinatumomab),6 costimulatory receptors (e.g., CD137, CD134, and toll-like receptor) agonists,7,8 and interferons/immunocytokines (e.g., interleukin-12).9 However, as indicated by increasing evidence, this cancer treatment suffers from a low response rate on several types of solid tumors, limited patient benefits, immune-related adverse events, and pseudoprogression.10,11Inspired by enormous benefits achieved by combined therapy, the combination of immunotherapy with radiation therapy (RT), termed as radio-immunotherapy, has recently thrived as a potent weapon fighting against cancer and a potential solution to the above concerns.12–14 Briefly, RT and immunotherapy share a solid biological basis for their synergistic antitumor effect. RT, consisting of external beam irradiation (X-rays, γ-rays, protons, or carbon ions) and internal radioisotope-mediated brachytherapy,15 is a prevalent cancer treatment method as nearly 50% of cancer patients have received RT.16 For antitumor therapy, RT exhibits a local tumoricidal effect and induces stromal, immunological, and vascular changes, but it often fails to treat metastases. In this context, systemic immunotherapy is an excellent addition to RT to achieve a whole-body anti-primary-tumor and anti-metastasis therapy. In addition, RT can evoke various immunogenic responses, particularly immunogenic cell death (ICD) and the “abscopal effect”.17 Thus, RT may act as a response enhancer for immuno-oncology treatments in a systemic immune-associated manner or a local therapy-focused manner. Specifically, multiple effects induced by RT, such as inducing in situ cancer vaccinations for tumor eradication and immune memory maintenance,18 overcoming the barrier of the dense tumor matrix and normalizing tumor vascular for easy access of ICIs and CAR T cells to the tumor tissue,19 or activating the cGMP-AMP synthase-stimulator of interferon gene (cGAS-STING) pathway for the recruitment of pro-inflammatory chemokines,20 have been found to be associated with an enhancement in the immune response. Furthermore, RT at a low dose has been demonstrated to help in normalizing the tumor vasculature, evoking the systemic immune response, and reprogramming the tumor stroma.21 However, high-dose RT is capable of causing severe blood vessel damage, overcoming a large tumor burden, and converting “cold” tumors to “hot” ones that are much more sensitive to immunotherapy.22,23 For example, brachytherapy at a high dose of 10 Gy was found to convert 80% of “cold” prostate cancer cells into “intermediate” or “hot” immune subtypes in 24 prostate cancer patients.24 Meanwhile, several studies have implied that immunotherapy can aid in prompting the “abscopal effect” induced by RT.25,26
Recent years have witnessed encouraging results from clinical trials of radio-immunotherapy; however, cancer radio-immunotherapy is hindered by three critical issues. First, the aggregate antitumor potency is still inadequate to treat a sum of indications, for example, dormant lesions in cancer patients and immunosuppressive tumor types.27 Second, innate or adaptive resistance to radio- and/or immuno-therapy, as well as these therapies-related adverse effects, has been reported.28,29 Third, there is a lack of valid criteria to help in guiding and assessing this radio-immunotherapy. For example, imaging manifestations, like hyperprogression and pseudoprogression, could display a false signal, leading to a significant delay in the treatment plan.30
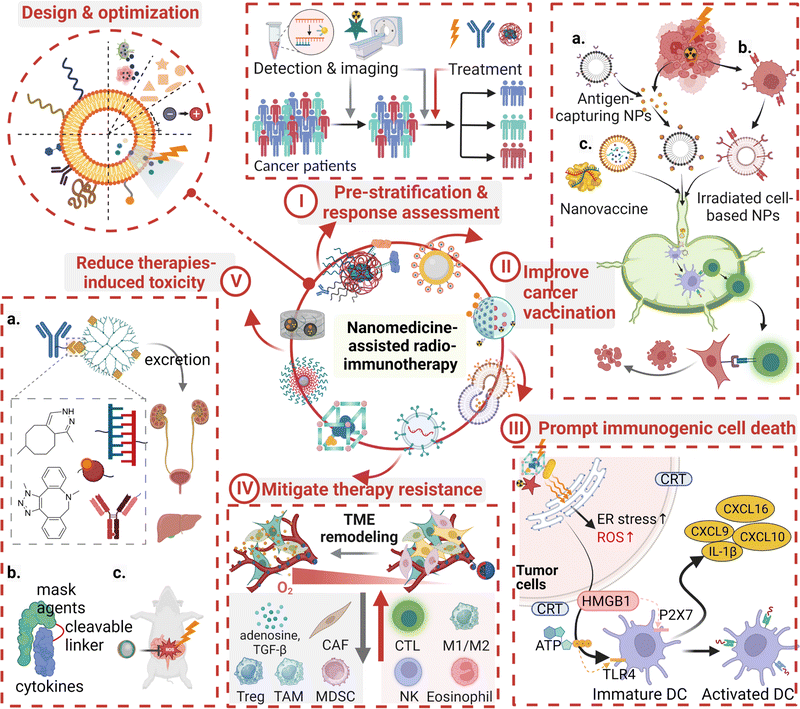
To address the above issues, nanomedicines have garnered increasing interest for cancer radio-immunotherapy due to their improved pharmacokinetics and incredible auxiliary functions (Fig. 1). Unique physicochemical properties and functional modifications endow these nanomedicines with the ability to overcome the obstacles encountered by conventional chemo- or immuno-therapeutic drugs, such as a low therapeutic drug concentration in the region of interest, non-specific distribution in healthy organs or tissues, and a rapid excretion rate.31–34 Effectiveness and a low-toxic profile of liposome- or albumin-based nanomedicines have been demonstrated in routine cancer treatment.35–37 A myriad of nanomedicines have been explored for radio-immunotherapy. Basically, they are designed to promote the interaction between RT and antitumor immunity. For example, in one aspect, nano-radiosensitizers augment local radiation deposition and improve radioresistance, resulting in increased ICD and in situ vaccination.38,39 In other aspects, nanocarriers with/without intrinsic bioactive properties have been applied to deliver therapeutic/diagnostic radionuclides, tumor antigens, ICIs, and immunomodulators to provoke strong, durable antitumor immunity, address immunosuppression, and realize imaging-aided therapy.40,41 Besides, incorporation of nanomedicines into adoptive cells or CAR T cells to deliver ICIs represents a clinical translational path for radio-immunotherapy.42 Encouragingly, nanomedicines have been applied in routine clinical practices and more in clinical trials or pre-clinical studies for radio-, immuno-, or combinational radio-immuno-therapy.
Overall, this review provides a comprehensive coverage of nanomedicine-assisted cancer radio-immunotherapy, including design strategies and synergetic antitumor mechanisms of nanomedicines for radio-immunotherapy. Their recent applications in solid tumors and considerations over their clinical translation have also been elaborated.
2. Cancer immunotherapy embraces radiotherapy
After years of rapid expansion of cancer immunotherapy, accumulative evidence indicates that the combinational therapies, particularly the integration of renascent immunotherapy with radiotherapy, have great potential to replace mono-immunotherapy and become the mainstream workforce in immuno-oncology-based treatments.43,44Non-irradiated remote tumor recession on patients who are subjected to RT is the clinical basis to develop cancer radio-immunotherapy. This phenomenon, termed as the “abscopal effect”, is well-known, but it is a low incidence event in clinical practice, mainly due to insufficient antitumor immune response elicited by RT and an immunosuppressive TME.45 In a recent clinical trial, about 29% of 168 patients with advanced tumors receiving anti-PD-1 and RT showed the “abscopal effect”.46 The underlying mechanism of this effect is complex and remains to be understood. Immunomodulatory signals or agents including DNA damage-induced inflammatory signals, released tumor neoantigens, and dendritic cell maturation stimulators have been found to contribute to the effect,47,48 but the effect was not seen in immunodeficient athymic mice that received RT.49 Thus, this effect confirms the immunomodulatory properties of RT and multiple biological processes are involved in the effect.
2.1 Biological synergistic basis of cancer radio-immunotherapy
The primary biological synergistic basis of cancer radio-immunotherapy is shown in Fig. 2, which includes the immunomodulating effect of RT and the radio-sensitization effect of immunotherapy.Additionally, a recent study based on murine tumor models and the cancer patient samples after treatment with RT and anti-PD-L1 immunotherapy revealed that both treatments shared a similar adaptive immune response by eliminating tumor-promoting erythroid progenitor cells (Ter cells), which secreted artemin to promote tumor growth.68 In summary, their synergistic effects on alleviating early-stage/late-stage small/large tumor burdens, curbing local foci/distant metastases, activating the antitumor immunity at dormant tumor sites, and preventing tumor recurrence can be harnessed for combating advanced cancers.69
2.2 Current landscape of cancer radio-immunotherapy
Recent years have witnessed the successful development of cancer radio-immunotherapy. Hundreds of clinical trials on this topic have been registered, and their recent progress has been summarized elsewhere.70,71 Various RT methods, such as external beam radiation therapy or brachytherapy with hypo- or hyper-fractionation at a high- or low-radiation dose using different radiation sources, have been combined with different immuno-oncology treatments including ICIs, CAR T, bsAbs, or cytokines via different modes of administration (i.v., s.c., i.p., and i.d.) sequentially or concurrently. The safety and efficacy of these combination treatments have been evaluated, and their indications include but are not limited to non-small cell lung cancer, glioblastoma, pancreatic cancer, soft tissue sarcoma, and prostate cancer. Herein, a few representative clinical trials are listed in Table 1.| Combinational strategies | Trial registration no. | Phase | Status | Indications | Enrollment | Arms description | Treatment outcome |
|---|---|---|---|---|---|---|---|
| Abbreviations: SBRT, stereotactic body radiation therapy; PFS, progression free survival; OS, overall survival; ORR, overall response rate; EBRT, external beam radiation therapy; CEA, carcinoembryonic antigen; SCLC, small cell lung cancer; HSG, histamine–succinyl–glycine; 153Sm-EDTMP, samarium 153 lexidronam pentasodium; TLR, toll-like receptor; CR, complete response; PR, partial response; SD, stable disease; SABR, stereotactic ablative body radiotherapy.a Represents the actual participants.b Indicates the estimated number (All data but therapeutic outcome were accessed from ClinicalTrials.gov). | |||||||
| RT + immune checkpoint inhibitor (ICI) | |||||||
| SBRT + durvalumab (αPD-L1) or tremelimumab (αCTLA-4) | NCT02311361 (REF72) | I/II | Completed | Unresectable pancreatic cancer | 65a | Four cohorts: durvalumab + 8 Gy × 1 f (A1) or 5 Gy × 5 f (A2), durvalumab + tremelimumab + 8 Gy × 1 f (B1) or 5 Gy × 5 f (B2) | Acceptable safety profile; modest treatment benefits: median PFS (1.7 vs. 2.5 vs. 0.9 vs. 2.3 months) and OS (3.3 vs. 9 vs. 2.1 vs. 4.2 months) in the cohorts A1, A2, B1, and B2, respectively |
| SBRT + pembrolizumab (αPD-1) | NCT02492568 (REF73) | II | Completed | Non-small cell lung cancer (NSCLC) | 92a | Two arms: pembrolizumab (A); SBRT (8 Gy × 3 f) + pembrolizumab (B) | Similar toxicity profile; clinical benefits: ORR (18% vs. 36%, p = 0.07), median PFS (1.9 vs. 6.6 months, p = 0.19), and median OS (7.6 vs. 15.9 months, p = 0.16) in the arm A and B, respectively |
| EBRT + vaginal brachytherapy + pembrolizumab | NCT04214067 | III | Recruiting | Stage I–II endometrial cancer | 168b | Two arms: brachytherapy + EBRT; EBRT + brachytherapy + pembrolizumab | Not available |
| RT + chimeric antigen receptor T (CAR T) cells | |||||||
| Yttrium-90 microspheres + anti-CEA CAR T | NCT02416466 (REF74) | I | Completed | Liver metastases | 8a | One arm: anti-CEA CAR T (three hepatic artery infusions) + Yttrium-90 microspheres | No grade 4/5 toxicity events; clinical benefits: median OS (6.9 months) |
| RT + CAR T | NCT04790747 | I/II | Recruiting | Hematological malignancies | 50b | One arm: sequential RT + intravenous infusion of CAR T | Not available |
| RT + bispecific antibody (bsAb) | |||||||
| 131I + omburtamab | NCT01099644 (REF75) | I | Active, not recruiting | Peritoneal cancer | 54a | One arm: intraperitoneal injection of 131I-8H9 (omburtamab) | No dose-dependent toxicities; transient adverse effect; phase II activity was established at 2.96 GBq m−2 |
| Anti-CEA × anti-HSG TF2 bsMAb + IMP-288-Luteium + IMP-288-Indium | NCT01221675 (REF76) | I/II | Completed | CEA-expressing SCLC or NSCLC | 18a | Two arms: optimization study and escalating activity phase I/II study | Absorbed doses predicted from pre-therapeutic imaging session for therapy session; a shorter pre-targeting delay (24 h) and the highest TF2 molar dose were the best parameters |
| RT + cancer vaccine | |||||||
| EBRT + sipuleucel-T | NCT01807065 (REF77) | II | Completed | Castrate-resistant prostate cancer | 51a | Two arms: sipuleucel-T (A); EBRT + sequential sipuleucel-T (B). | Both arms were well-tolerated; median PFS (2.46 vs. 3.65 months, p = 0.06) in the Arm A and B |
| Radium-233 + sipuleucel-T | NCT02463799 (REF78) | II | Completed | Prostate cancer | 36a | Two arms: radium-233 + sipuleucel T (A); sipuleucel-T (B). | No synergistic toxicity; median PFS (10.7 vs. 3.1 months, p = 0.02), PSA response (33% vs. 0%, p = 0.04), and AlkPhos response (60% vs. 7%, p = 0.01) in the arm A and B, respectively |
| 153Sm-EDTMP + vaccine | NCT00450619 (REF79) | II | Completed | Prostate cancer | 44a | Two arms: 153Sm-EDTMP radiation (A); 153Sm-EDTMP + recombinant fowlpox- and vaccina-TRICOM vaccine + sargramostim (B). | Both arms have similar toxicity; median PFS (1.7 vs. 3.7 months, p = 0.034) and a >30% PSA decline (0 vs. 19%, p = 0.073) in the arm A and B, respectively |
| RT + autologous dendritic cells (DCs) | |||||||
| EBRT + autologous DCs | NCT01347034 | II | Completed | Soft tissue sarcoma | 20a | Two arms: EBRT alone; EBRT + autologous DCs (i.t.). | One in fourteen had serious adverse events in the treatment group with EBRT + DC injection; therapeutic outcome is not given. |
| RT + DC immunization + temozolomide | NCT03548571 | II/III | Recruiting | Glioblastoma | 60b | Two arms: DC immunization (i.d.) + subsequent RT (2 Gy × 30 f) and temozolomide; RT (2 Gy × 30 f) and temozolomide | Not available |
| RT + costimulatory receptor agonist | |||||||
| RT + GLA-SE (TLR4 agonist) | NCT02180698 (REF80) | I | Completed | Metastatic sarcoma | 16a | One arm: glucopyranosyl lipid A-stable-emulsion (GLA-SE) + RT | Grade 1 or 2 toxicity; local tumor control (14/14), and 1 CR, 1 PR, and 11 SD on 156 days post-trial |
| RT + imiquimod (TLR7 agonist) | NCT01421017 (REF81) | I/II | Completed | Breast cancer with chest wall recurrence or skin metastases | 31a | Three arms: RT + imiquimod; RT + cyclophosphamide; RT + cyclophosphamide + imiquimod | All but one adverse event was grade 1 or 2; abscopal response was generated in 3/9 cases |
| RT + interferon/immunocytokine | |||||||
| SBRT + L19-IL2 | NCT02086721 (REF82) | I | Completed | Oligometastatic solid tumors | 6a | One arm: SBRT + subsequent L19-IL2 (i.v.) | Recommended dose in phase II is 15 million international Units |
| SABR + Darleukin (L19-IL2) | NCT03705403 (REF83,84) | II | Recruiting | Stage IV NSCLC | 126b | Two arms: standard of care SABR and/or RT; SABR and/or RT + L19-IL2 | Not available |
Beyond these selected clinical trials, analysis results of two large-datasets provide a current summary of the therapeutic outcomes in the combinational treatment of immunotherapy and RT. An analysis of the National Cancer Database reveals that the addition of immunotherapy to RT contributes to an improved overall survival time (OS) in melanoma brain metastases according to the data from 1104 patients. 192 patients received both RT and immunotherapy with a median OS of 11.1 months, while the rest were treated with RT alone with a median OS of 6.2 months.85 However, a retrospective analysis of patients with metastatic NSCLC, among which 6383 received RT plus immunotherapy and 170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 479 received hypofractionated RT alone, reveals no difference in the overall survival time between two groups.86 Thus, this combined therapy displayed encouraging therapeutic outcomes for a few cancer types, but it is not effective against other indications. Additionally, several issues, including ambiguity between pseudoprogression and tumor progression, the off-target effect of immune-adjuvants and radio-enhancers, and therapeutic resistance, contribute to under-expected therapeutic efficacies and severe systemic toxicities.
479 received hypofractionated RT alone, reveals no difference in the overall survival time between two groups.86 Thus, this combined therapy displayed encouraging therapeutic outcomes for a few cancer types, but it is not effective against other indications. Additionally, several issues, including ambiguity between pseudoprogression and tumor progression, the off-target effect of immune-adjuvants and radio-enhancers, and therapeutic resistance, contribute to under-expected therapeutic efficacies and severe systemic toxicities.
3. Nanomedicines aid in cancer radio-immunotherapy
3.1 Basic mechanisms
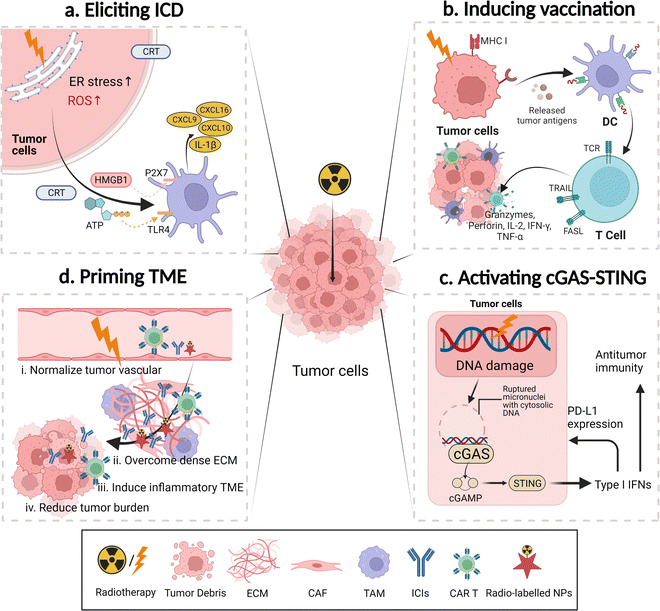
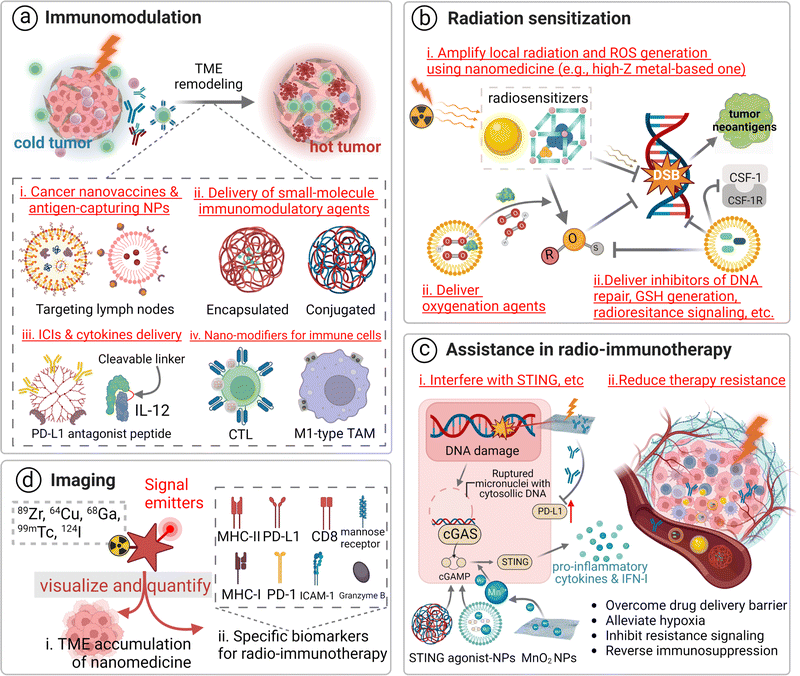
Overall, application of nanomedicines improves the safety and efficacy of cancer radio-immunotherapy. Potential roles of nanomedicines in this combined therapy can be divided into four categories: immunomodulators, radiosensitizers, regulators for improving both therapies, and probes for diagnosis, therapeutic response evaluation, or imaging-assisted treatments. In this combinational treatment, nanomedicines may play multiple roles through incorporation of different functional groups (Fig. 3).In cancer radio-immunotherapy, there are four main specific immunomodulatory roles of nanomedicines. 1. They act as cancer nanovaccines or antigen-capturing NPs. Briefly, exosomes or irradiated cell membranes of RT-treated tumor cells are used as personalized cancer nanovaccines due to the abundance of tumor neoantigens and DAMPs in them. The use of antigen-capturing NPs can help in adsorbing and presenting released antigens after RT, favoring DC activation and enhancing antigen-specific T cell-mediated antitumor immune response.89,90 2. They deliver immunomodulatory agents in the manner of physical encapsulation or chemical conjugation.91–93 Agonists of STING and TLR, as well as inhibitors of indoleamine-2,3-dioxygenase (IDO), have been incorporated into the nanoscale delivery system, contributing to evading immune escape or potentiating ICI-based antitumor immune response for RT.94,95 3. They deliver ICIs and cytokines. Inefficient delivery and unpleasant systemic toxicity of immuno-drugs hamper their clinical application, while nanotechnology may help in addressing these issues.96–98 For instance, the result of a phase II clinical trial (NCT00396019) with 86 participants indicates the administration of PEGylated interferon Alfa-2b (PEG-Intron) to patients with plexiform neurofibromas could significantly delay the onset of tumor progression in comparison to the placebo group.99 Additionally, advanced nanotechnology-aided immunomodulatory nanomedicines have been developed, such as PD-L1 antagonist peptide-decorated polymeric nanoparticles,100 a dendrimer–ICI conjugate,101,102 and a cytokine or an antibody modified with an inner stimuli-responsive mask agent.103,104 4. They can be used to modify immune cells. Nanomedicines, mostly in the form of nanogels, can be modified to attach to effector T cells or macrophages via a backpacking approach to simultaneously realize multiple functions, such as controlled release of IL15 to selectively expand tumor-infiltrated T cells or IFN-γ to maintain proinflammatory M1 macrophages.105,106
There are three major means of realizing nanomedicine-aided radiation sensitization: (i) amplifying local radiation deposition and ROS generation by using high-Z nanomedicines. Metal elements and their nano-derivatives with a high atomic number and efficient nano-catalytic properties, particularly in the form of metal–organic frameworks, including hafnium oxide, gadolinium, gold, bismuth, platinum, polyoxotungstate, titanium oxide, and tantalum, have been explored for this radiation sensitization effect.38,108–110 For example, hafnium oxide-containing nanoparticles (NBTXR3) have been evaluated in 15 clinical trials and they have shown a promising therapeutic effect. The working principle of this nanoparticle is dose partitioning. An increased radiation dose is deposited close to this high-Z radiosensitizer, resulting in improved photoelectric interaction.111 In addition, the high-Z radiosensitizer can contribute to non-oxygen-dependent ROS generation by catalyzing a great amount of H2O2 in the TME. Furthermore, mitochondria- or endoplasmic reticulum-targeting nanomedicines could contribute to highly-selective damage to tumor cells;112–114 ii. Delivering tumor hypoxic radiosensitizing reagents. Hypoxia is a major contributor to radio-resistance. Thus, tumor re-oxygenation strategies, including the use of oxygen-saturated nanomaterials (perfluorocarbon),115 oxygen-generators (MnO2),116 NO-releasing prodrugs,117 HIF-1α inhibitor-containing carriers,118 catalase, catalase-like metals or H2O2,119 have been employed to address tumor hypoxia-induced radioresistance. iii. Carrying inhibitors for DNA repair and radioresistance signaling. Inhibitors for vital proteins (mTOR) and signaling pathways (hedgehog signaling or CD73/adenosine) have been incorporated into nanomedicines to indirectly increase ROS deposition and augment the microenvironmental stress.120,121 Inhibitors for DNA repair (olaparib),122 enhancers for RT-induced apoptosis (perifosine),123 and disrupters for the cell cycle (proflavine hemisulfate, gemcitabine, and pentoxifylline) and the NAD+ metabolism are the family members of radiosensitizers,124,125 as they directly or indirectly improve the therapeutic outcome of RT.
In the first approach, the STING pathway is one of the most studied pathways. Generally, nanomedicines can be used to increase RT-induced ruptured micronuclei with cytosolic DNA, as well as deliver exogenous STING agonists, which augment the STING activation for a robust antitumor immune response. For instance, cGAMP/MOL, comprising a STING agonist cGAMP and an Hf12-Ir metal organic layer, elicited robust STING activation with low-dose radiation. Significant response from interferon regulatory factors and excretion of STING-IFN axis-related inflammatory cytokines (IFN-β and IL-6) were observed after treatment with the nano-radiosensitizer compared to the one with cGAMP alone.126 In another study, an exogenous STING agonist, c-di-AMP, was integrated into a Mn2+ chelated tannic acid-based nanoplatform to treat large tumors. The level of the second messenger of STING, cGAMP, was significantly increased by 2-fold in the 4T1 tumor tissue in this nano-combinational treatment group in comparison with that in the RT-treated one on day 12 post-treatment.127
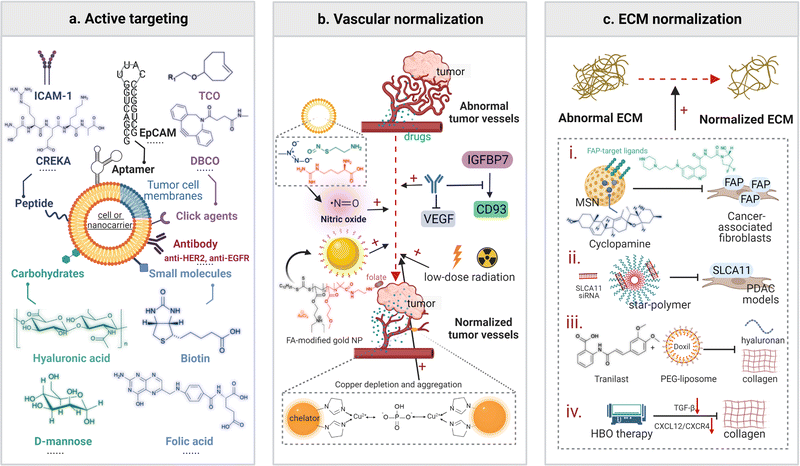
Strategies for overcoming physiological barriers during nano-medicinal delivery and addressing hypoxia, a main factor conducive to the resistance to radio-immunotherapy, have been employed to improve the therapeutic efficacy of radio-immunotherapy. Briefly, modification with active targeting moieties, vascular normalization, and ECM normalization are three common nanomedicine-assisted TME-modulating methods. Tumor re-oxygenation to address hypoxia is achieved via exogenous delivery of oxygen-generating agents or endogenous generation promoted by delivered reagents.
In contrast to current clinical routine imaging modalities, nanomedicine-assisted imaging is devoted to improving the sensitivity and specificity of imaging signal of tumors undergoing radio-immunotherapy. Internal/external radiation exposure may alter the immune phenotype of tumors and specific biomarkers on the cell surface (such as PD-1, PD-L1, and CTLA-4) or the related cells (cancer-associated fibroblast and tumor-associated macrophages) may have thereafter been changed.129,130 This dynamic process can be monitored with the help of engineered site-specific probes, which consist of corresponding targeting moieties and imaging moieties. Multi-functional probes labelled with different signal sources could be constructed thanks to advances in nanotechnology.131 They can be designed to reach multiple targets or deliver multiple imaging agents to obtain a comprehensive coverage of the disease status. A dual positron emission tomography (PET)/near-infrared fluorescent probe, which was prepared from 89Zr, near-infrared fluorophore (CF-MPTMS)-attached silica nanoparticles with protamine on the surface and a heparin coating layer, was used to in vitro label CAR T cells and in vivo track these cells in mice models bearing ovarian peritoneal carcinomatosis.132 Many ex vivo cell labelling methods, such as radionuclide-labelling via copper-free click chemistry,133 and superparamagnetic iron oxide labelling,134 have been established to visualize immune effector T or NK cells during the treatment process.135
Unfortunately, there is no well-defined, accurate biomarker yet for cancer radio-immunotherapy. A few critical indicators, such as CD8,136 granzyme B,137 and lymphocyte activation gene-3,138 show positive correlation between their expression and the therapeutic outcome. Additionally, the use of imaging probes for multiple biomarkers could be a promising solution for combination therapy. Challenges remain for interpretation of these images and correlation of these imaging signals with the exact therapeutic response to this combination treatment. Support of evidence-based medicines and the use of artificial intelligence in medical imaging analysis could help in addressing these challenges.139
3.2 Design strategies of nanomedicines for radio-immunotherapy
Elegant and rational design of nanomedicines is a critical step for their application in radio-immunotherapy. Clinical requirements, preparation, and delivery routes for these nanomedicines should be considered.Current landscape. Formulations of nanomedicines and their potential role in the current practice of this combined therapy are selected and shown in Table 2. The roles of these nanomedicines are very different depending on their formulations and indications. They can be a nanocarrier for an exogenous antigen (OVA) or a radiosensitizer to improve radiosensitivity and overcome radiotherapy resistance, a scaffold for immunomodulator drugs, or a container for in situ generated neoantigens. As shown in Table 2, these nanomedicines not only offer therapeutic benefits, but also act as an adjuvant medium to enhance radio-immunotherapy.
| Ref. | Nanoformulation | RT | IT | Comments |
|---|---|---|---|---|
| Abbreviations: IT, immunotherapy; IDO-1, indoleamine 2,3-dioxygenase-1; APPs, Au-Pt@PCN-224; RIT, internal radioisotope therapy; CTLs, cytotoxic T lymphocytes; AHSC, atovaquone, hafnium, sabutoclax, and chlorin Ce6-containing metal-phenolic networks; RGD-EV, cyclic RGDyK peptide-modified extracellular vesicles; HPV, human papillomavirus; R-DOTAP, R-enantiomer of 1,2-dioleoyl-3-trimethylammonium-propane chloride; DMPG, dimyristoyl phosphatidylglycerol; DPPC, dipalmitoyl phosphatidylcholine. | ||||
| Preclinical studies | ||||
| Organic | ||||
| Zhang et al.140 | Smac-TLR7/8 peptide | γ-ray EBRT | TLR7/8 agonist | This peptide self-assembled into a nanofibrous and porous hydrogel, overcoming the radioresistant TME and repolarizing TAMs |
| Li et al.141 | AmpFY9 peptide | γ-ray EBRT | IDO-1 inhibitor | This pH-responsive transformable peptide aided in reversing immunosuppression via the GSH-responsive release of NLG919 |
| Inorganic | ||||
| Wang et al.95 | 4PI-Zn@CaCO3 | X-ray EBRT | IDO-1 inhibitor | Neutralizing tumor acidity via biocompatible biomineral CaCO3 and depleting Kyn via 4PI potentiated RT against CT26 and 4T1 tumors by inducing potent antitumor immune response |
| Dong et al.142 | WO2.9-WSe2-PEG NPs | X-ray EBRT | αPD-L1 | Semiconductor-based nanoscale radiosensitizers combined with αPD-L1 in a triple-therapy manner to treat local and distal tumors |
| Organic–inorganic hybrid | ||||
| Pei et al.143 | 177Lu-APPs-PEG | RIT | RT-induced immune response | The radioactive nano-oxygen generator relieved tumor hypoxia, promoted CTL infiltration, and inhibited tumor cells and Tregs |
| Sang et al.144 | AHSC nanoparticles | X-ray EBRT | αCTLA-4; αCTLA-4 + αPD-L1 | NP-reinforced RT and αCTLA-4 increased PD-L1 expression, promoting anti-PD-L1 therapy |
| Biomimetic | ||||
| Tian et al.145 | PD-L1 siRNA loaded RGD-EV | X-ray EBRT | PD-L1 siRNA | An in situ EV-mediated ICI therapy was applied to glioblastoma with short-burst radiation |
| Qin et al.146 | Au@MC38 | X-ray EBRT | αPD-1 | Live MC38 cell-derived biogenetic AuNPs were formed; these Au@MC38 NPs displayed homologous targeting of tumor cells, thus enhancing RT and immune response |
| Clinical trials | ||||
| Liposome | ||||
| NCT04580771 | Liposomal HPV-16 E6/E7 Multipeptide Vaccine | EBRT | Vaccine | This phase II trial aimed to improve the therapeutic response against HPV-infected cervical tumors by using a liposomal vaccine (six HPV-16 peptide-containing lipid R-DOTAP) |
| NCT00828009 | BLP25 liposome vaccine | EBRT | Vaccine | This phase II trial aimed to treat lung cancer with a liposomal vaccine (lyophilization of BLP25 lipopeptide, monophosphoryl lipid A, and three lipids: cholesterol, DMPG, and DPPC) and bevacizumab after EBRT |
| PEG | ||||
| NCT04936841 | NKTR-214 (PEGylated IL-2) | EBRT | Immuno-cytokine and αPD-1 | This phase II trial aimed to deliver PEGylated IL-2 and αPD-1 in combination with RT to treat head and neck cancer |
| Protein | ||||
| NCT04756505 | Bintrafusp Alfa | EBRT | Fused protein and NHS-IL12 | A fused protein composed of αPD-L1 and TGF-beta, an immunocytokine, and the protein was combined with RT to treat breast cancer in the phase I trial |
| Inorganic nanoparticles | ||||
| NCT05039632 | Hafnium oxide-containing nanoparticles (NBTXR3) | EBRT | αCTLA-4/αPD-1 | This phase I/II trial investigated the adverse effects and therapeutic benefits of a combined therapy, consisting of NBTXR3-augmented RT, αCTLA-4, and αPD-1 for metastatic solid tumors |
Advances in nanoformulations for nanomedicines. A library of cancer nanomedicines for immunotherapy or radiotherapy has been extensively summarized elsewhere.147,148 This review concentrates on advances in nanoformulations for nanomedicines that promote radio-immunotherapy.
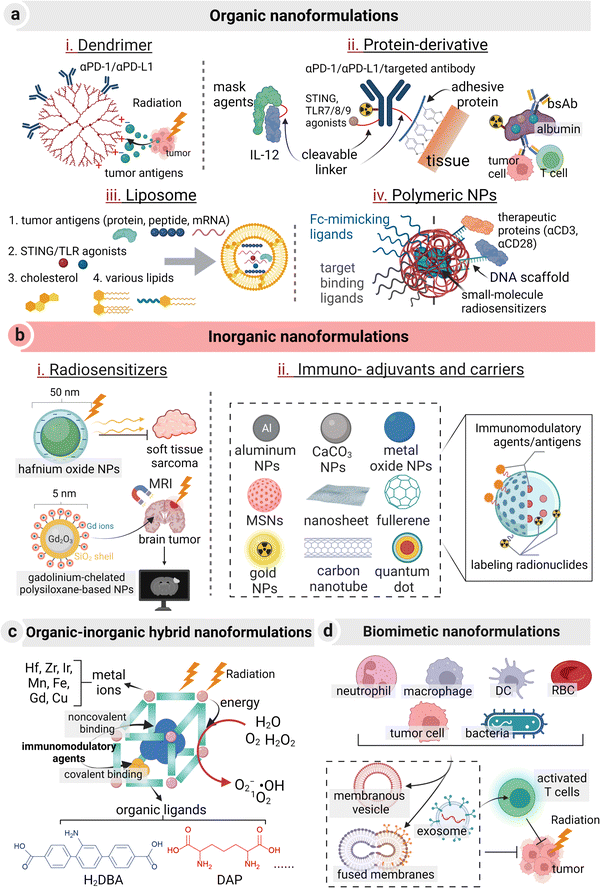
The incorporation of a multi-functional nanocarrier into the nanomedicine for cancer radio-immunotherapy has led to the maximum therapeutic effect with reduced toxicity to a large extent. Four major types of nanocarriers have been explored for these nanoformulations: organic, inorganic, organic–inorganic hybrid, and biomimetic nanocarriers (Fig. 4). The classification is established on the basis of the body structure of the nanoformulation. To note, biomimetic nanoformulations refer to those biological products originating from mammalian cells or bacteria, while natural or synthetic proteins are considered as organic nanoformulations.
Liposomes, polymers, and albumin are the most widely used organic nanocarriers in clinical drug delivery practice due to their non-immunogenic or low-immunogenic properties and great biocompatibility.149 In the preclinical studies, dendrimers and natural polysaccharides have recently gained popularity compared to other polymers. Specifically, multivalent binding properties of dendrimers can be harnessed to increase the binding kinetics of ICIs to their corresponding cell surface receptors.150 In a recent study, hyperbranched G7 poly(amidoamide) dendrimers were conjugated with αPD-L1, with an estimate of 3.7 ± 0.5 antibodies per one dendrimer and a one-order-of-magnitude increase in the binding kinetics with PD-L1 in comparison with free αPD-L1.101 Natural polysaccharides, particularly hyaluronic acid, stand out for their immunomodulatory effects.97,98 Notably, several hydrophobic immunological agents or radiosensitizers, such as cyclic dinucleotides and 2-(2-nitroimidazol-1-yl) acetic acid, can act as the hydrophobic domain in self-assembled polymeric conjugates.151,152
Protein-derivatives are another essential source of organic nanomedicines,153 which can be divided into protein-based nanocarriers, protein-based therapeutics (e.g., antibody or antigenic peptide), TME-modulating enzymes, and multifunctional hybrid proteins.154 For protein-based nanocarriers, mussel adhesive proteins have recently emerged as an efficient nanocarrier for localized stable retention of anti-PD-L1 because of their substantial tissue-adhesion properties.155 Besides, their size plays a critical role when they are delivered in a systemic manner. In contrast to dimeric-nanobodies (anti-HER2 2Rb17c-2Rb17c, control R3B23-R3B23, and dimeric monovalent 2Rb17c-R3B23) and mAb (trastuzumab), monomeric nanobodies (2Rb17c) turned out to be the most effective one in homogenous intratumoral distribution and rapid renal clearance.156 In the regime of protein-based therapeutics, fragments or entire antibodies/antigens can be engineered with nanomedicines. For example, tumor antigens and Fc fragments were fused to the C-terminal of the protein nanocarrier to form a cancer nanovaccine, enabling DC activation and tumor-specific targeting.157 In addition, bispecific T-cell engagers (BiTEs) were nano-engineered with two single-chain variable fragments (scFvs) that targeted T cells and tumor cells, respectively.158,159 Similarly, synthetic nanoparticle antibodies were prepared from a Janus nanoplatform with a cell-targeting ligand on one “face” and a Fc-mimicking ligand on the opposite “face”.160 Both BiTEs and synthetic nanoparticle antibodies were engineered onto the surface of the nanomedicine with multivalent contact. In addition, a short-chain synthetic DNA scaffold was demonstrated as a versatile tool to optimize the surface of an organic nanocarrier or a cell to strengthen the effect of their immunomodulation on immune cells.161 In a previous study, via complementary DNA reaction, different therapeutic protein molecules (anti-CD3 and anti-CD28, IL-2, or synthetic priming antigens) were ratiometrically loaded onto the surface of poly(lactic-co-glycolic acid) (PLGA) polymer–DNA nanoparticles. Encouragingly, this biotechnology realized intact bioactivity of protein molecules, in vivo CAR T activation and tumor clearance via an “AND” logic-gate, and ex vivo T cell activation and expansion.162
Inorganic nanoformulations have been widely used in clinical practice or trials, including aluminum salt (aluminum hydroxide and aluminum phosphate), graphene or silica. They act as cancer vaccine adjuvants for antigen reservoir and DC maturation,163 as well as radiosensitizers. NBTXR3 (phase III, status: recruiting) and AGuIX (phase II, status: recruiting) are two examples of inorganic nano-radiosensitizers.39 In preclinical studies, a great sum of inorganic nanomedicines, such as aluminum NPs, CaCO3 NPs, metal oxide NPs (e.g., MnO2), and mesoporous silica NPs, emerge as potent radiosensitizers, immuno-adjuvants or carriers. These inorganic nanomedicines exhibit high Z metal- or hypoxia relief-mediated radiation sensitization, display inherent or acquired immunomodulatory effects, and possess a high drug-loading capacity.164,165 In the term of radiation sensitization, several high-Z element-based nanomedicines with efficient nano-catalytic properties, such as hafnium oxide, gadolinium, gold, bismuth, platinum, and titanium oxide, have been reported to augment radiation deposition and ROS generation.108–110,166 Besides, these inorganic nanomedicines can be doped or labelled with radionuclides or self-assembled from radionuclides themselves (such as β-particle emitter 198Au) as an internal radioisotope therapeutic agent.167 Several radiolabeling methods have thereafter been prompted. A recent study proposed a universal chelator-free radiolabeling method, i.e., the use of a SnCl2/HCl solution and Tween 80 for labelling therapeutic 188Re with both inorganic (SiO2, Au, and Fe3O4) and organic (PLA) NPs with a high labelling efficiency up to 98% and an in vitro radiochemical stability of 95%.168 Their adjuvant roles in immunotherapy are generally divided into two types. One is adsorption or delivery of antigenic peptides or mRNA for a sustained effect.169 The other one is to realize immune-stimulation by their biological active structures. For instance, PEGylated mesoporous silica nanoparticles with a tunable pore diameter and a large internal surface have been previously reported to trigger the TLR4/NF-κB pathway in macrophages and recruit T cells to inflame cold tumors.170,171 β-alanine-modified Gd@C82 was also demonstrated to reprogram TAMs to the tumor-killing M1 type.172 Notably, upon external or internal stimulation, metal oxide-based nanoparticles can neutralize tumor acidity or catalytically decompose over-expressing H2O2 to relieve the immunosuppression in the TME (e.g., CaCO3 or MnO2) for enhancing the RT efficacy,95,173,174 or generating adequate ROS to induce in situ vaccination (e.g., HfO2).175 More importantly, with the rapid progress of biodegradable strategies in designing inorganic nanomedicines, their toxicity issues related to long-term retention and tough clearance can be elegantly addressed.176
Metal–organic frameworks (MOFs), consisting of high Z metal ions/clusters and coordinated organic molecules, are a typical organic–inorganic hybrid nanocarrier used in radio-immunotherapy. They have enjoyed a blooming growth in this field due to their homogeneous porous structure, the radiosensitization effect, and their therapeutic enhancements in vaccination and ICI treatment.177,178 For example, cationic nMOFs were applied to deliver anionic CpGs via electrostatic interactions to perform X-ray-activated vaccination.179 A wide range of metal ions/clusters and coordinated organic ligands in preparing MOFs endow this hybrid nanocarrier with a diversity of functions. For instance, the high-Z element Hf or Bi has the radiation amplified effect, metals (Mn, Fe) are able to act as an imaging and immune- or redox-modulating agent, and organic ligands such as Ir(DBB)[dF(CF3)ppy]2+, Ir(bpy)[dF(CF3)ppy]2+, DBA, and DBP (corresponding chemical nomenclature: DBB, 4,4′-di(4-benzoato)-2,2′-bipyridine; dF(CF3)ppy, 2-(2,4-difluorophenyl)-5-(trifluoromethyl)pyridine; bpy, 2,2′-bipyridine; DBA, 2,5-di(p-benzoato)aniline; DBP, 5,15-di(p-benzoato)-porphyrin) are photosensitizing.180–182 A study has witnessed more than 99% of tumor regression in a MC38 mice model treated with Hf-based nMOFs at a low radiation dosage of 0.5 Gy × 5 fractions. Mechanistically, the secondary building units (SBUs) of electron-dense Hf12 and Hf6 absorbed X-rays to produce hydroxyl radicals. The generated energy was transferred to the photosensitizing ligand Ir(DBB)[dF(CF3)ppy]2+ to produce 1O2 and O2−.183 Monte Carlo simulation results revealed that the radiosensitization effect of lattices in the MOF exceeded solid NPs due to enhanced scattering of photons and electrons within the lattices regardless of the radiation source and particle size. Thus, tuning the lattice parameters, such as the SBU size and/or the inter-SBU distance could contribute to an optimal radiation dose.184 Meanwhile, other inorganic–organic hybrid nanocarriers, such as organic ligands, polymers or biomacromolecule-modified inorganic nanoformulations could be potential candidates of radio-immunotherapy.
Biomimetic nanocarriers have the reputation for their biocompatibility, the intrinsic tumor-homing effect, and inherent immunogenic properties.185–187 A myriad of engineered biomimetic membrane-based vesicles (single membrane-based vesicles, fused cell membrane-based vesicles, and exosomes), originating from blood cells, platelets, tumor cells, immune cells, and bacteria, have been utilized to deliver antigenic agents or other therapeutic agents to elicit an immune response in the practice of cancer vaccination or radio-immunotherapy.188–192 These biomimetic nanocarriers provide a strong shelter to prevent premature leakage and enzymatic lysis of cargos such as nucleic acids or proteins. Among single membrane-based vesicles, one typical and prevalent application is to use tumor cell membranes or bacteria-derived outer membrane vesicles (OMVs) as a biomimetic coating layer to realize targeted delivery of ICIs or radiosensitizers, as well as immune-activation.193,194 Fused cell membrane-based vesicles integrate characteristic biochemical receptors and surface functional groups of each individual cells, and thereafter can act as a potent nanocarrier to achieve multiple functions in radio-immunotherapy. For example, fusion of the cytomembranes of mature DCs and tumor cells achieved co-expression of tumor antigens and immunological costimulatory molecules.195 Other hybrid vesicles have also been widely explored, including fusion of membranes from erythrocytes and tumor cells,196 hybridization of the membranes of macrophages and cancer cells,197 or blending of autologous tumor cell membranes and OMVs.198 And recently, liposomes/lipid reagents or polymeric nanostructures have been introduced to cell membrane-based vesicles to improve their stability and address their issues, such as size- or shape-controllability and reproducibility.199,200 Lipid-bilayer extracellular vesicles are also employed to enhance cell–cell communication, strengthen their interaction with non-cellular TME constituents, and deliver bioactive cargos.201,202 HER2+ extracellular vesicles from BT-474 cells were successfully attached to the surface of MDA-MB-231 cells from triple-negative breast cancer tissues. This tissue has no therapeutic receptors, and the introduction of extracellular vesicles into these cells helped in achieving targeted therapy of HER2 positive cancer.203 Immunomodulatory drugs including BDC-1001 (TLR7/8 agonists) and pembrolizumab (PD-1 antibody), as well as HER2-targeting radionuclide drugs (e.g., 131I-GMIB-Anti-HER2-VHH1, 177Lu-DOTA-ADAPT6-ABD035), have been employed in this case.204–207
Overall, the above four major types of nanocarriers, particularly liposomes, protein-derivatives, inorganic radiosensitizers, and biomimetic vesicles, have great potential in clinical translation in consideration of their drug-loading capacity, biological safety, encouraging preliminary clinical trial results, and acceptance by patients and doctors. Meanwhile, the interaction between nanocarriers and biological cells, immunotoxicity, and immunogenicity remain to be understood.208 Thus, intensive studies on these aspects of nanocarriers should be investigated. For instance, graphdiyne oxide as a nanocarrier helped polarizing M2 macrophages to pro-inflammatory ones.209,210
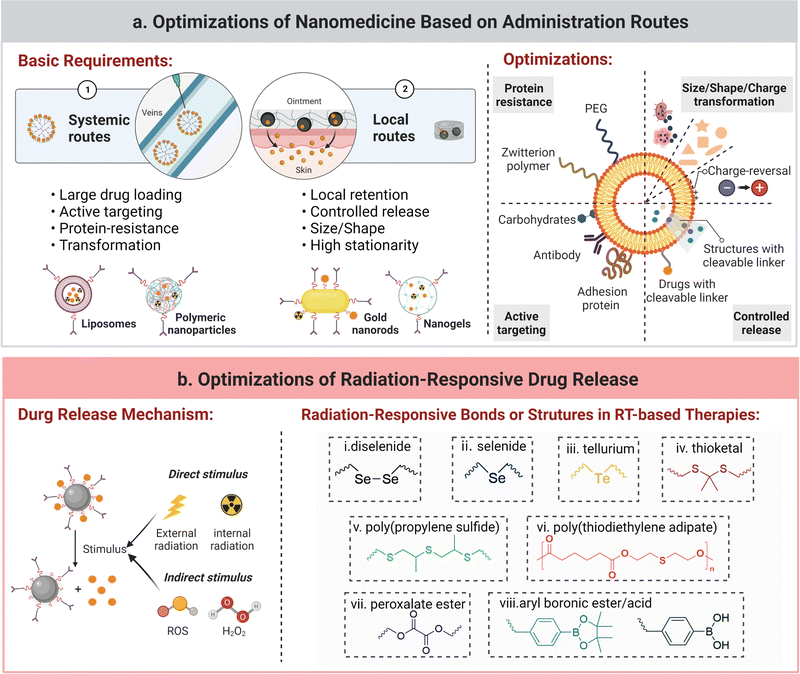
Optimization of nanomedicines. Optimization of nanomedicines can be obtained by modulating their size, shape, elasticity, composition, surface charge, and surface chemistry.213,214 In cancer radio-immunotherapy, size-changeability and shape-deformability were found to play an important role in delivering antitumor vaccines to lymph nodes or immunoregulatory agents to tumors.215 It was reported that a rod-like shape of mesoporous silica nanoparticles outperformed in targeting tumor cells, including MCF7 cells and pancreas cancer cells (PANC-1 cells), rather than healthy cells (MCF10A and CAFs) in comparison with the spherical one.216 For tuning the surface charge, a charge-reversal modification strategy by harnessing a tumoral acidity-responsive bond that links a positively-charged inner ligand and a neutral or negative anti-fouling outer ligand (e.g., zwitterion polymer or PEG) has been applied to construct a nanomedicine with negligible surface protein adsorption and high tumor cellular uptake.217 In the case of lymphatic transport, a negatively-charged nanocarrier was found to be superior to the positively-charged one.218 Moreover, active surface modifications with sufficiently accessible ligands enhance selective targetability of nanomedicines towards tumor cells or tumor-associated macrophages.219,220 In one study, surface modification of glucose moieties in generation 4 hydroxyl PAMAM dendrimers endowed them with the targetability towards TAMs via interaction between glucose and glucose transporters. Meanwhile, replacement of glucose with galactose facilitated targeting galactins on the surface of glioblastoma cells.221 In addition, layer-by-layer (LDL) surface modification is an emerging method to simultaneously allow specific targeting and toxicity mitigation of high-dose immunocytokine therapy. Single-chain interleukin-12 was coated onto a liposome, and the prepared product was wrapped with a second layer of poly-L-arginine and a third layer of poly-L-glutamic acid or hyaluronic acid. Even at an increasing dose, a significant toxicity reduction and an enhanced therapeutic efficiency were observed in the treatment of this LDL-modified nanoparticle toward murine colorectal and ovarian tumors.222
To conclude, the optimization of size, shape, structure, surface charge or surface chemistry of nanomedicines can endow them with the ability of loading onco-immunological agents (tumor antigens, STING agonists, or ICD inducers) and radio-enhancers to tumors or lymph nodes in a more safe and effective manner.223–225
Additionally, nanomedicines can be optimized according to their systemic or local administration routes. A high level of drug accumulation in lesions, stability during blood circulation, and a low level of distribution in normal tissues are essential for nanomedicines in a systemic administration manner. Thus, nanomedicines are often tuned or modified to have a high drug loading, specific tumor-targetability, and resistance to protein corona formulation. Meanwhile, for local administration of nanomedicines via intratumoral injection, transdermal administration, transarterial embolization, or portal vein embolization, they should have a prolonged residence time with controlled release of antitumor drugs including immunotherapeutic agents or radiosensitizers in the region of interest.226 Strategies including size-tunability for local retention and chemical conjugation for prolonged retention are preferable in the design of these nanomedicines. For instance, in a recent study, thermal-responsive phase transition of elastin-like polypeptides and electrostatic interaction between their oligolysine tail and CpG were utilized to promote the formation of a local complexation depot, achieving long-term local retention of GpG for more than 3 weeks and therapeutic 131I for 2 weeks.227
Optimization of stimuli-responsive drug-release from nanomedicines. Stimuli-responsive drug release from nanomedicines triggered by external stimuli (ultrasound, magnetic, light, or X-ray radiation) or internal stimuli (low pH, high ROS, and over-expressed proteases in the TME) can help in releasing immunotherapeutic agents or radiosensitizers at the target tumor site and reducing their toxicity to normal tissues.228–232 External therapeutic radiation can be an excellent external stimulus for controlled release of tumor antigens and immunotherapeutic agents in radio-immunotherapy.50 For instance, PhotoCORM MnBr(CO)5, a photo-sensitive moiety to release CO in a lanthanide scintillator NP (ScNPs:NaLuF4:Gd,Tb@NaLuF4), was indirectly activated by external X-rays to subsequently realize the release of the CO gas in a deep-tissue (about 5 cm) and simultaneously achieve CO-mediated ROS generation and ICD.233 The common radiation-responsive chemical bonds or structures are summarized in Fig. 5b. The underlying working principle of radiation-responsive drug release is not well understood and different mechanisms have been proposed, for example, radiation triggered photolytic degradation of a PLA polymer.234,235 In another case, γ-rays triggered the cleavage of hydrogen bonds between the antitumor drug, pemetrexed, and cytosine-containing diselenide in diselenide-pemetrexed assemblies, and the released drug activated NK cells and exerted its antitumor activity.236 ROS-sensitive bonds can also respond to γ-ray radiation since they induce ROS generation. For example, hydrophobic poly-(propylene sulfide) was reported to be oxidized to hydrophilic sulfoxides/sulfone in the presence of •OH that was generated upon γ-ray radiation, and a robust release of encapsulated drugs was achieved during the oxidation process.237
Optimization of administration routes for nanomedicines. The administration routes of nanomedicines into the body can be categorized into systemic delivery (intravenous or oral administration) and localized supply (intra-tumoral, intra-nodular, transdermal, intra-peritoneal, intranasal, or intraocular administration). As reported, the ability of inducing vaccination via intradermal/subcutaneous injection, intramuscular injection, and i.v. injection/oral administration of nanomedicines is in the descending order.238
The optimal administration route for a nanomedicine is heavily dependent on its therapeutic mechanism and the characteristics of indications.239 It is suggested that cancer nanovaccines should reach lymphatic sites to augment the presentation of antigens to APCs, the maturation of APCs, and the activation of antigen-specific cytotoxicity T cells. There are two major routes for delivery of nanomedicines into lymphatic sites: direct access through parenteral injection (subcutaneous and intradermal), or mucosal administration (enteral, pulmonary, and intravaginal).240 The sequence of administration is essential to the final vaccination effect. In a recent study, simultaneous or sequential intravenous/subcutaneous (IV/SC) vaccination was carried out using antigen/CpG-loaded layered double hydroxide nanoparticles in a tumoral murine model bearing E.G7-OVA-lymphoma or B16F10-melanoma. The antitumor effect of IV-priming + SC-boosting was much stronger than that of the untreated group, and more than 75–90% of the tumor volume shrank after sequential IV/SC vaccination. Simultaneous IV/SC injection of this cancer nanovaccine contributed to a one-week delay of tumor progression to the end point when the tumor volume reached 1000 mm3 in both tumor models at an early-stage with an initial volume of 50 mm3 and a late-stage with an initial volume of 500 mm3, respectively.241
Intensive studies have been devoted to establishing and selecting in vitro/in vivo models for evaluating the therapeutic potency of nanomedicines for radio-immunotherapy. Ideal models should be able to map the actual tumor microenvironment in the human body, including the immune system. Discoveries from these models could accelerate clinical translation. While, in reality, a cancer tissue consisting of tumor cells and at least one immune cell population from a systemic-stimulated mouse model (genetically-engineered or therapy-induced) are often selected to determine the therapeutic efficacies of radio-immunotherapy.
Generally, animal tumor models consist of subcutaneous- or orthotopic-grafted cancer, metastasis-induced cancer (intravenous or intracardiac engraftment), and artificially induced spontaneous cancer.248 The orthotopic animal model offers a biomimicking tumor microenvironment similar to that of the original cancer development. A transgene-driven model may provide a great insight on early oncogenesis and tumor mutation-induced neoantigens, while a carcinogen-driven model can display constitutional heterogeneity in tumor tissues.249 Notably, the selection of the mouse species is a crucial parameter in evaluating the effectiveness of cancer radio-immunotherapy. It has been found that C57BL/6 mice prefer to developing Th1 immune response, which is vital to CD8+ T cell-participated antitumor immune response.250 Hence, C57BL/6 mice species is widely used for evaluating the efficacy of radiotherapy and immunotherapy. The ratio of M1/M2 phenotype of TAMs varies among mice models and it is higher in the model bearing 4T1 murine breast cancer than the model with CT26 tumors.118 In addition, these cancer models should be reproducible and practical to be built. Tracking of the cell lineage could be essential to realize reproducibility.251 In the preclinical trial of treating cancer with radio-immunotherapy, bilateral tumor models and re-challenged tumor models are the most frequently used. To develop a re-challenged model for a prophylactic nanovaccine against cancer or pre-immunization, the nanovaccine or an immune-stimulation agent is primarily injected into the left and right footpads of mice models. After seven-day immunization, cancer cells are subsequently injected.252 It has been reported that the efficacy of the vaccine depends on the survival rate of the injected cancer cells. Additionally, thanks to rapid progress of microfluidic chips, in vitro silico-based models, such as patient-specific glioblastoma models, have been established as a robust predictive tool for radio-immunotherapy.253
However, there are very few reliable, reproducible and cheap models covering metastasis, cancer prevention, tumor dormancy or quiescence, and immune- or radiation-resistance. More importantly, due to inherent limitations, the animal models used in most pre-clinical studies on radio-immunotherapy are murine cell line-derived models, not human tumor-originated ones. Three emerging humanized murine models for immune-oncology-based therapy, including the Hu-PBL model, Hu-CD34 model, and BLT model, have been built on immunodeficient mice via injection of human peripheral blood mononuclear cells (PBMCs), human CD34+ hematopoietic stem cells, and human fetal liver and thymus along with stem cells, respectively.254 However, these humanized models suffer from a few issues: induction of a severe graft-verse-host disease, inability to induce MHC-restricting tumor antigen-specific immune response, and a complex modeling process.255 Efforts should be devoted to building models to capture accurate and dynamic biological information of human cancer tissues while maintaining accessibility so that the potency and biosafety of nanomedicine-assisted caner radio-immunotherapy can be properly and widely assessed.
4. State-of-the-art nanomedicine-assisted cancer radio-immunotherapy
Nanomedicines have assisted in cancer radio-immunotherapy in two major aspects. One is that nanomedicines have aided in in vitro diagnosis, in vivo pre-selection, real-time monitoring, and evaluation of the therapeutic response of cancer patients. They have also contributed to increasing the therapeutic efficacy and reducing toxicity. Their state-of-the-art advances are summarized in Table 3.| Main function | Interventions | Remarks | Ref. |
|---|---|---|---|
| Abbreviations: IO, iron oxide; aiMRI, activatable inflammation magnetic resonance imaging; OVA, ovalbumin; SPG, Shirasu porous glass; RP@RMs, irradiated cancer cell membrane coated on R837-loaded PLGA; Phy@PLGdH, physcion@layered gadolinium hydroxide; GDYO, graphdiyne oxide; NIA, 2-(2-nitroimidazol-1-yl) acetic acid; AuDAP, dual-functional Au nanoparticle; CLIO, cross-linked dextran iron oxide. | |||
| Pre-stratification and response assessment | 64Cu-labeled polyglucose NPs | Tumor associated macrophage imaging; | 256 |
| Monitoring TAM response to adjuvant therapy; | |||
| Correlating imaging intensity with TAM densities. | |||
| 68Ga-NOTA-Nb109 | Nonblocking imaging of PD-L1. | 257 | |
| ROS-responsive IO-Gd nanovesicle | Early stratification of RT response; | ||
| aiMRI approach is developed; | |||
| Acute oxidative stress bridge antitumor immunity. | 258 | ||
| Nanovaccine and in situ cancer vaccination | Bi2O3@OVA@DC + RT | OVA as a synthesis template for Bi2O3 NPs; | 259 |
| Improved efficiency compared with OVA@DC; | |||
| Augmenting the STING signaling by Bi2O3 NPs. | |||
| PLGA/CpG@PDA-Au + RT | Rapid SPG membrane emulsification; | 89 | |
| Radio-sensitization; | |||
| In situ capture of RT-induced antigens. | |||
| RP@RMs | Irradiated tumor cell membranes as a vaccine; stronger immunogenicity of RMs. | 54 | |
| Prompting ICD | Phy@PLGdH nanosheets + RT + αPD-L1 | Shape affecting radiation deposition; | 260 |
| Nanosheets outperforming spherical one; | |||
| Inhibiting the pentose phosphate pathway. | |||
| H@Gd-NCPs + RT + αPD-L1/αCTLA-4 | Radiation deposition & GSH depletion; | 261 | |
| Decomposition of H2O2 by Hemin; | |||
| Sensitized RT potentiating ICI therapy. | |||
| Hf-CpG MXF + RT | CpG as DNA components of MXF; | 262 | |
| Maintaining long-term immune-memory. | |||
| Overcoming therapy resistance | GDYO nanosheets | Inherent immunomodulatory properties; | 210 |
| Polarizing M2- to M1-type TAMs; | |||
| Stimulating NF-κB & MAPK pathways. | |||
| NIA-D1@R848 + RT | NIA reducing radioresistance of hypoxia cells; DPPA-1 relieving suppression of T cells. | 263 | |
| AuDAP + RT | Dual targeting with AS1411 aptamer & M2pep; | ||
| Repolarizing M2 to M1 via NF-κB signaling axis. | 264 | ||
| Reducing therapy-induced toxicity | Masked IL-12 | Protein domain as a mask agent; | 96 |
| Tumor protease-cleavable linker; | |||
| Encouraging efficacy without systemic irAEs. | |||
| Three click-antidote: phospholipid-PEG micelles, BSA, CLIO NPs + click-antibody | Ab-antidotes derived from NPs; | 265 | |
| Short-circulating CLIO NPs perform better than other two clicked NPs and CLIO nanoworms. | |||
| Polydopamine NPs | Oral administration; | 266 | |
| Scavenging ROS and suppressing inflammation; curing RT-induced intestinal injury. | |||
4.1 Nanomedicine-assisted imaging for radio-immunotherapy
Imaging cancer tissues is an integral part of cancer treatment and it has been realized through a wide range of nanomedicines. For cancer radio-immunotherapy, nanomedicines have effectively helped pre-selecting patients for this treatment, assessing therapeutic response of patients during the treatment, and identifying immune-related adverse events (irAEs). Furthermore, in vivo imaging biomarkers of cancer tissues or tumor-infiltrating immune cells could provide guidelines for preparing nano-formulations to achieve effective radio-immunotherapy in pre-selected patients.Nanomedicine-assisted imaging strategies for pre-stratification of patients include: (a) imaging the expression of biomarkers that are associated with positive therapeutic outcomes, (b) monitoring dynamic changes in biomarkers upon therapeutic interventions at a stimulating dose for rapid pre-evaluation of a treatment plan, and (c) assessing the accumulation level of therapeutic agents in the tumor tissue.269 These obtained images can help in initially discriminating responders from non-responders towards radio-immunotherapy. Among all imaging modalities, nuclear medical imaging techniques, such as PET and single photon emission computed tomography (SPECT), have ultra-high imaging sensitivity and are a preferred choice for imaging in radio-immunotherapy.270 In detail, radionuclides emit β+ or γ rays for PET or SPECT imaging signal, respectively, as well as β− or α rays for radiation therapy. These radionuclides can be chelated to a nanomedicine to diagnose and pre-stratify cancer patients simultaneously.271 For instance, PET imaging of intercellular adhesion molecule-1, an up-regulated inducible glycoprotein in non-irradiated tumors in mice receiving RT, can be a valuable tool to pre-select patients with the “abscopal effect” at an early stage.272
A sum of factors, such as a high tumor mutational burden, T cell-inflamed gene expression, PD-1/PD-L1 expression, mutations in the repair or correction pathways for DNA damage or mismatch, and microsatellite instability, have been taken into consideration as reliable clinical biomarkers, as well as independent indicators to jointly stratify human cancer to some extent.273–275 In a recent report, an anti-hPD-L1 heavy chain-only antibody, Nb6, was labelled with a PET signal-emitting radionuclide, 124I, to select positive-responsive responders in an osteosarcoma OS-732 tumor for the following anti-PD-L1-based immunotherapy. This Nb6 antibody with a high affinity for hPD-L1 was first selected from 95 monoclones with the help of phage display technology. 124I-anti-hPD-L1 displayed a high binding affinity value (2.19 nM) towards OS-732 cells in vitro and an accumulation amount of 4.43 ± 0.33% ID g−1 in the OS-732 tumor tissue at 24 h post-injection in vivo, confirming its biological effect on its application in bioimaging.276 In another study, 64Cu-labelled polyglucose nanoparticles, termed as Macrin, were constructed for quantitative analysis of TAMs via imaging; this probe with a size of ∼20 nm exhibited a high selectivity (>90%) towards macrophages. Since the amount of a radionuclide-labelled model drug accumulated in TAM-rich tumors was more than 7-fold that of TAM-deficient tumors, this TAM-monitoring approach showed a potent ability of pre-selecting patients (Fig. 6a).256 In whole, imaging PD-L1 expression on tumor cell and the infiltration density of TAMs is currently the most feasible pre-stratification strategy for immuno-based therapy.
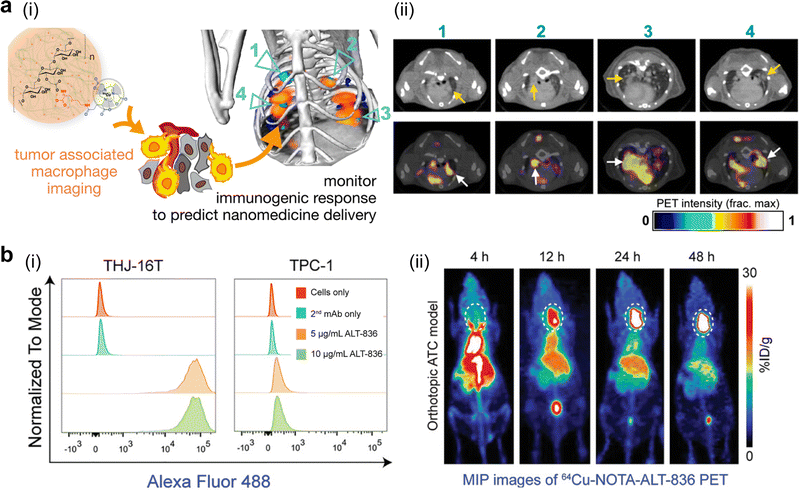 | ||
| Fig. 6 Pre-stratification of murine tumor models through nanomedicine-enabled PET imaging of tumor-associated biomarkers. (a) 64Cu-labelled polyglucose nanoparticles were constructed for quantitative analysis of tumor-associated macrophages: (i) the chemical structure of this probe and its working principle; (ii) PET/CT imaging of KP lung adenocarcinoma-bearing C57BL/6 mice at 24 h post-injection of this probe. Reproduced with permission. Ref. 256 Copyright 2018, American Chemical Society. (b) A 64Cu-labelled tissue factor-specific mAb was developed to image tissue factors: (i) flow cytometry confirmed a high level of the tissue factor in THJ-16 anaplastic thyroid cancer (ATC) cells; (ii) representative maximum intensity projection (MIP) images of orthotopic ATC murine models using 64Cu-NOTA-ALT-836. Reproduced with permission. Ref. 277 Copyright 2020 the Authors, Published by Wiley-VCH. | ||
Tumor biomarkers in different cancer types, such as tissue factors and L1-cell adhesion molecules, have also been explored as promising pre-selection targets in assessing the accumulation level of nanomedicines at the tumor sites. The tissue factor (TF) was chosen as a potential target for anaplastic thyroid cancer. ALT-836 as a TF-specific mAb was thereafter developed. According to quantitative flow cytometry analysis, ALT-836 displayed a much higher level of cellular uptake in TF-abundant THJ-16T cells than TF-deficient TPC-1 cells. Its radionuclide-labelled derivative, 64Cu-NOTA-ALT-836, was reported to achieve high accumulation in subcutaneous and orthotopic ATCs; the peak of tumor uptake reached 19.93 ± 2.17% and 37.20 ± 1.71% ID g−1 in subcutaneous and orthotopic models, respectively (Fig. 6b).277 The L1-cell adhesion molecule (L1CAM) in cholangiocarcinoma was investigated in another study. A diagnostic radioisotope, 64Cu, was conjugated to NH2-terminated chimeric anti-L1CAM (cA10-A3) through a bifunctional chelator, 2-S-(4-isothiocyanatoenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA). In vivo biodistribution of 64Cu-NOTA-cA10-A3 (37 MBq/100 μg) peaked with 18.9 ± 2.6% ID g−1 at 48 h post-injection at the SCK-L1 tumor site, while its tumor accumulation was much lower in other mice tumor models (Choi-CK, SCK, and JCRB1033). The in vivo imaging result was in line with the in vitro cell-bound assay and the L1CAM expression level in these cell lines, which was confirmed from western blotting and flow cytometry analysis, indicating the feasibility of using this 64Cu-NOTA-cA10-A3 probe for detecting L1CAM-positive patients.278
In summary, nanomedicine-assisted imaging strategies broaden the way to pre-select patients for nanomedicine-based radio-immunotherapy. Moreover, classification methods to distinguish tumor immune states have emerged, such as transcriptomic-based analytical platforms for TME subtypes and tumor immunity in the microenvironment.279,280 These methods may dramatically accelerate the discovery of specific and sensitive biomarkers for pre-stratification of cancer patients and facilitate the development of their corresponding nanomedicine-assisted imaging probes.
A clear, early understanding of tumor response to therapies in holistic cancer treatment is essential to improve clinical outcomes. The nanomedicine-assisted in vitro diagnosis approach and in vivo functional imaging emerge as powerful and timely tools for evaluating and predicting body response to radio-immunotherapy. Because of a relatively slow therapeutic response towards radiotherapy and immunotherapy due to changes in tumor size, invasion, and metastasis, this nanomedicine-mediated approach can unveil timely, accurate and specific information about tumor tissues after RT treatment, such as dynamic changes in immune cell infiltration and early indicators (e.g., elevated ROS or caspase-3).258,282
Circulating tumor DNA (ctDNA) acts an early evaluation indicator for in vitro diagnosis and provides valuable prognostic information about patients treated with antitumor immunotherapy and/or radiotherapy.283,284 A feasible approach for the in vitro detection of ctDNA could be the use of nanomedicine-aided detection probes, such as gold nanoparticles and DNA nanomedicines.285 However, the prognostic value from in vitro detection is restricted due to a lack of unified criteria for sampling time, sampling location, multi-biopsy, and tumor heterogeneity,286 while in vivo nanomedicine-mediated imaging is free from these limitations. It can provide accurate immune profiles of patients and its non-invasive imaging features cause less harm to the body.
PD-L1 (∼33 kDa) is recognized as a well-known biomarker for radio-immunotherapy. After RT intervention, it is highly expressed in tumor cells and antigen-presenting cells to realize immune escape, which may facilitate αPD-L1 therapy.20,287 A diagnostic radionuclide 89Zr-conjugated anti-PD-L1 (atezolizumab), was utilized in a recent clinical study to image 22 patients. Each patient had one unique tumor and there were three different tumor types among 22 patients including metastatic bladder cancer, non-small cell lung cancer, and triple-negative breast cancer. Compared to immunohistochemistry (IHC)- or RNA-Seq-based in vitro predictive biomarkers, the generated PET signal from this 89Zr-labelled mAb was better correlated with the therapeutic responses.288 In a previous pre-clinical study, 89Zr-desferrioxamine(Df)-atezolizumab was employed for non-invasive quantitation of PD-L1 expression after RT treatment. Two lung cancer cell lines, PD-L1+ H460 cells and PD-L1− A549 cells, were selected in this study. After delivering external RT (5 fractions of 2 Gy) to tumor sites, tumor uptake of 89Zr-Df-atezolizumab in PD-L1+ H460 bearing mice was significantly increased, while its uptake was slightly improved in mice models with PD-L1− A549 cells. Of note, this RT treatment significantly improved the PD-L1/β-actin ratio by nearly six fold in H460 cells. These results suggested that the PD-L1 expression was upregulated after RT, and this 89Zr-labelled antibody could be used to monitor the change in PD-L1 expression.289 Moreover, some engineering strategies haven been developed for PD-L1 targeting. In one report, a radionuclide-labelled lipid-PD-L1 aptamer was applied for the immunoimaging of PD-L1. A 99mTc-labelled and PD-L1 aptamer-modified C18 chain (C18-apPDL1) was constructed and exhibited two-fold tumoral accumulation at 24 h post-injection (about 0.88% ID g−1) compared to the PD-L1 aptamer and a random sequence-modified C18.290 Notably, nonblocking imaging of PD-L1 on tumor cells, a novel and advanced imaging method, emerges and allows real-time monitoring of PD-L1 expression without interfering with the PD-L1 blockade-based therapy. 68Ga-NOTA-Nb109, composed of a specific single-domain antibody with a different binding epitope from that of anti-PD-L1 and a chelated PET tracer (68Ga-NOTA), was applied to quantitatively assess PD-L1 expression in tumors. Successfully, this probe had a relatively high equilibrium dissociation constant of 2.9 × 10−9 M and a maximum uptake ratio of 5.0 ± 0.35% ID g−1 could be achieved in A375-hPD-L1 tumors at 1 h post-injection (Fig. 7a).257 Therefore, PD-L1 peptide antagonists that do not affect the binding of PD-L1 antibodies could be explored to modify imaging probe-labeled or non-labeled immunomodulatory nanomedicines after RT treatment.
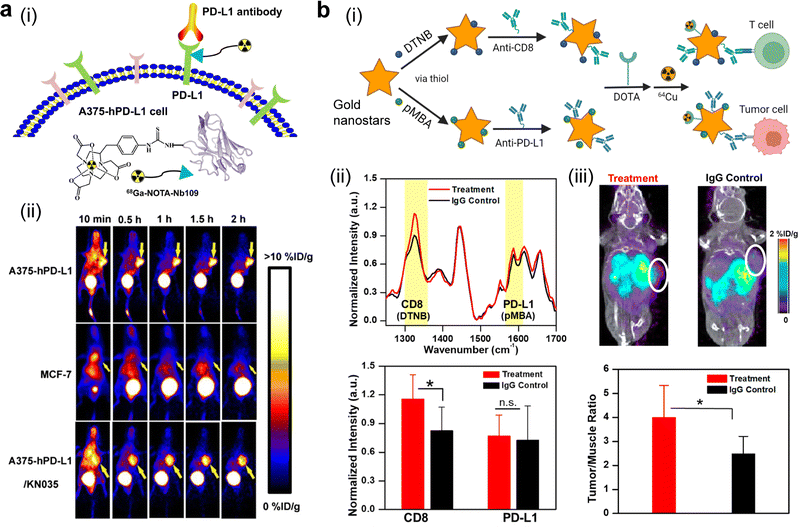 | ||
| Fig. 7 PET imaging via nano-probes aids in accurate and specific monitoring of therapeutic responses towards immune-related therapies. (a) Nonblocking PET imaging of PD-L1 using 68Ga-NOTA-NB109: (i) illustration of the nano-probe and its working principle; (ii) dynamic PET imaging of three murine tumor models using this nano-probe. Reproduced with permission. Ref. 257 Copyright 2020, Society of Nuclear Medicine and Molecular Imaging. (b) Dual-model surface-enhanced Raman spectroscopy (SERS)/PET imaging of PD-L1 and CD8+ in immuno-oncology-treated YUMM2.1 tumors via an engineered immunoactive probe prepared from gold nanostars: (i) scheme of the preparation process of this nano-probe; (ii) SERS spectra of murine tumor models receiving anti-CD137 + anti-PD-L1 treatment and an IgG control, as well as their corresponding SERS quantification; (iii) PET-CT imaging of the treated groups at 24 h post-injection of this nano-probe and their corresponding PET quantification. Reproduced with permission. Ref. 291 Copyright 2019, American Chemical Society. | ||
Other emerging technologies, including multiple biomarker-targeting multimodal probes and radiomic analysis, allow biomarker-driven predictions of tumor response to radio-immunotherapy. A dual PET/SERS imaging nanoprobe was used to simultaneously detect PD-L1+ tumor cells and CD8+ T cells (Fig. 7b). A Raman reporter, 5,5-dithiobis(2-nitrobenzoic acid) with a peak wavenumber at 1325 cm−1 or para-mercaptobenzoic acid with a feature peak at 1580 cm−1, was incorporated on the surface of gold nanostars through a thiol–Au reaction. Correspondingly, PEGylated anti-CD8 or anti-PD-L1 was then conjugated to gold nanostars, followed by DOTA chelator conjugation and 64Cu chelation. After seven days from the initial treatment of both PD-L1 and CD137 agonists, two immunoreactive gold nanostars (IGNs) were injected to monitor the response towards immunotherapy. This treatment was repeated three times and the administration of IgG was set as a control. PET/CT imaging revealed a relatively high level of tumor accumulation of IGNs in the combinational treatment group (0.58% ID g−1) compared to that in the IgG-treated control group (0.31% ID g−1). Furthermore, the averaged SERS spectral intensity in the treated and control groups supported that much more CD8+ T cells were seen in the treatment group, which was consistent with the immunohistochemistry staining result via 3,3′ diaminobenzidine, an agent for CD8+ T cell-specific immunostaining. In all, this imaging platform provides a versatile scaffold for multiple biomarker-specific imaging.291 In another study, novel radiomic analysis based on quantitative imaging after incorporation of iodixanol-encapsulated liposomes was employed to assess myeloid-derived suppressor cell (MDSC)-directed immunotherapy. A prolonged blood circulation time and a high level of nanoparticle accumulation in perivascular regions facilitated spatiotemporal visualization of MDSC-influenced vascular structures. Furthermore, nanoparticle-mediated CT images were extracted for radiomic analysis, and the results indicated that the texture-based feature was helpful in differentiating treatment groups.292 Inspried by these findings, it could be inferred that dual targeting of MDSCs or M2-like TAMs could be valuable to determine the therapeutic resistance at the tumor site. Dual targeting strategies to inhibit these cells have been demonstrated to improve the antitumor therapeutic efficay,293,294 and depletion of both of them was reported to poteniate the ICI therapy,295 indicating the benefit of imaging both MDSCs and TAMs.
Pseudoprogression and hyperprogression are two typical outcomes in radio-immunotherapy, which are multiple factor-driven and not fully understood yet. It is reported that the overall incident rate of pseudoprogression is less than 10% in cancer patients receiving ICIs, for instance, 6.4% in patients with melanoma, 5% in patients with NSCLC, and 7% in patients with genitourinary cancer.296 Five major hypotheses are proposed for pseudoprogression, including (a) Treg expansion; (b) T-cell exhaustion; (c) modulation of pro-tumorigenic immune subsets; (d) oncogenic-pathway activation; and (e) aberrant inflammation.297 Meanwhile, hyperprogression refers to two distinct pathophysiologic phenomena: (1) rapid tumor progression may not be correlated with immune checkpoint inhibition; and (2) accelerated tumor growth may be induced by ICI-related premature death (e.g., apoptosis).298 With regard to its multiplex imaging and specific-targeting properties, nanoprobes may be an effective strategy to aid in the non-invasive discrimination until more relevant biomarkers have been found.
Overall, these advanced technologies, including in vitro diagnosis of circulating biomarkers or in vivo non-invasive advanced imaging tools, can provide a precise and clear landscape of tumor response to radio-immunotherapy. Nevertheless, one should note that not all these indices have clinical significance and some of them can only be applied to disease-specific evaluation. Meanwhile, a few principal endpoints such as overall survival, progression-free survival, and time to form new metastases may be overestimated for radio-immunotherapy, and other endpoints for radio-immunotherapy should be explored for better evaluation of the therapeutic outcome.
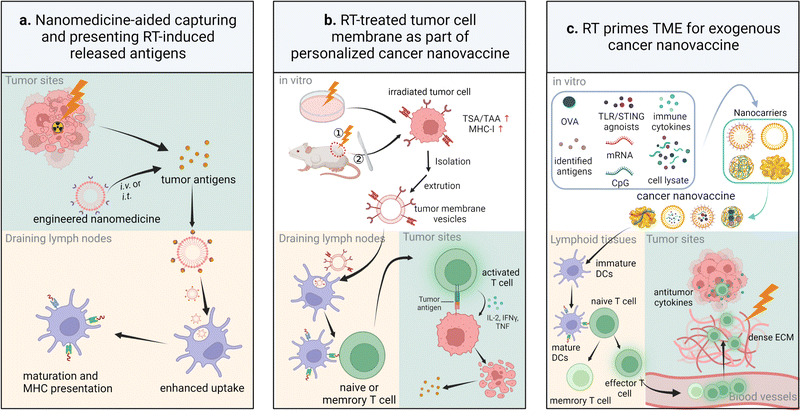
4.2 Cancer nanovaccine-participated radio-immunotherapy
Cancer nanovaccine-mediated vaccination, including exogenous vaccination, in situ cancer vaccination (ISV), or both, has emerged as a hot topic in cancer research to eradicate residual cancer tissues after surgical treatment, induce tumor regression and durable antitumor immune response, and prevent tumor progression.299,300 Currently, three major categories of clinically-available personalized cancer vaccines are whole cell lysates of DCs, synthetic long peptides, and mRNA.301 So far, there are two clinically-approved therapeutic cancer vaccines: Bacillus Calmette-Guerin (BCG) for early-stage bladder cancer and Sipuleucel-T (Provenge) for prostate cancer, and a few preventive cancer vaccines against HPV-induced cancer (Cervarix, Gardasil, and Gardasil-g) or hepatitis B virus-induced cancer (HEPLISAV-B).302 The nanomedicine-based biological negative or biological active vaccination adjuvants, which are beyond conventionally-used liposomes or lipidoid nanoparticles, along with RT, a potent inducer of ISV,303 have been demonstrated to boost innate or adaptive antitumor immune response and increase the therapeutic efficacy by improving antigenicity and adjuvanticity.304–307 Their working principle is concisely demonstrated in Fig. 8.Exogenous cancer vaccination is realized by cancer nanovaccines that carry model antigens or identified antigens. These nanovaccines carrying identified antigens are ready for large-scale production and clinical translation since these biological active agents are well understood. In contrast, individualized in situ vaccination relies mainly on the antigens from immunogenic dying tumor cells induced by radiation therapy or other therapies.308 Treatments, such as radiotherapy, Bacillus Calmette-Guerin, TLR agonists, oncolytic viruses, immune cytokines, and Doxil, can elicit in situ cancer vaccination.309 Compared with exogenous cancer nanovaccines, this in situ vaccination approach can avoid a complex preparation procedure, possible contaminations, and immune tolerance induced by the use of universal tumor-associated model antigens. Notably, antigen-capturing nanoparticles, such as maleimide-modified dendrimers or bacteria outer membrane vesicles and dopamine-coated PLGA polymeric nanoparticles, aid in forming in situ cancer vaccination by capturing antigens released from the above therapy-treated tumors.89,90
Several typical nanomedicines derived from polymers, MOFs, tumor-derived membrane-based vesicles or exosomes, or radionuclide-labelled ones are presented below and their potent vaccination effects are discussed.
Polymers, including micelles, polymer–epitope conjugates, and lipid–polymer hybrids, are the leading nanocarriers for cancer vaccination apart from liposomes. A pH-responsive PC7A nanovaccine, consisting of E7 or OVA antigenic peptide-loaded polyethylene glycol-b-poly(2-hexamethyleneimino ethyl methacrylate), was developed by Gao et al. for treating a sizeable solid tumor by incorporating local RT (20 Gy). In the tumor mice model of TC-1 (murine lung cancer) and B16-OVA (murine melanoma cancer), this combined treatment exhibited a significant volume reduction in both primary and distal tumors via systemic and local STING-activation mechanisms.310 Their following study on a library of melanoma antigens (Trp1, Tnpo3, Obsl1, Pbk, and Gp100) revealed that phase transition of these antigen structures from random coils to α-helix contributed to efficient antigenic peptide loading in PC7A micelles, which may provide a new means of selecting peptide antigens for nanovaccines.311 In another study, this PC7A polymer was applied to combine with CpG, while maleimide-decorated bacterial membrane was coated on them to form an in situ vaccine. This antigen-capturing nanoparticle aided in absorbing released neoantigens from tumor tissues after RT intervention and then cross-presenting to lymph nodes for subsequent DC maturation and CTL infiltration.312 In summary, the tunable physiochemical properties of polymer-based nanovaccines allow improving antigen transportation to immune follicles or tumors and protecting antigens from pre-degradation or rapid clearance.
A high-Z element-containing MOF is a potent nanostructure candidate for boosting exogenous and in situ cancer vaccination, which is primarily attributed to its porous and hollow structure for holding antigens or immune-stimulating agents, as well as its radio-sensitization effect to aid in RT-induced in situ vaccination.313 Two nanoscale MOFs, DBP-Hf and DBA-Hf, were prepared from an Hf cluster and a porphyrin-based photosensitizer ligand, 5,15-di(p-benzoato)porphyrin (DBP) and 5,10,15,20-tetra(p-benzoato)porphyrin (DBA), respectively. These prepared MOFs were applied to deliver an INCB024360 analogue, an IDO inhibitor, to realize synergistic radio-immunotherapy via an in situ vaccination approach. Both nanomedicines, IDOi@nMOF, exhibited distinctive radiation enhancement factors: 2.13–3.36 for DBP-Hf and 1.47–1.66 for DBA-Hf, in comparison with 1.00–1.22 for HfO2 at the same Hf concentration. Intratumoral injection of the IDOi-loaded DBP-Hf MOF in combination with low-dose X-rays (0.5 Gy × 6 fractions) to treat bilateral murine breast cancer (TUBO) and colorectal cancer (CT26) demonstrated a superior antitumor effect.177 In a similar study, anionic CpG was loaded on the surface of a cationic nMOF with a photosensitizing ligand via electrostatic interaction to obtain Hf-DBBF-Ir@CpG. Intratumoral injection of this nanomedicine and local X-ray irradiation were applied to a primary tumor during the treatment process. In situ vaccination was initiated by CpG incorporated in Hf-DBBF-Ir@CpG as a pathogen-associated molecular pattern agent and released agents (DAMPs and tumor neoantigens) from tumor cells induced by this treatment. Consequently, this approach efficiently activated antigen-presenting cells and facilitated the infiltration of cytotoxic T cells in tumor regions in immunosuppressive murine colorectal MC38 and pancreatic Panc02 models. When this treatment was combined with αPD-L1, the cure rate reached 83.3% in a T cell-absent MC38 model.179 One major advantage of MOFs is easy and broad modulation of their compositions (metal ions, organic ligands, and loaded agents) to realize different purposes. In a recent study, MRI-visible Mn2+ was chosen as metal ions, meso-2,6-diaminopimelic acid (DAP), a nucleotide oligomerization binding domain 1 agonist, was selected as organic ligands, and ovalbumin (OVA) acted as a model antigen. The combination of these compositions achieved an imaging-guided nanovaccine delivery to B16-OVA melanoma tumors.314
Tumor cell membranes or tumor-derived exosomes isolated from X-ray irradiated tumor cells could be employed as personalized nanovaccines due to their upregulated expression of MHC-I, IFN-γ, and tumor neoantigens.54,315 For instance, subcutaneous injection of microvesicles at an average size of 339.9 ± 139.0 nm produced from irradiated C6 glioma cells at a single dose of 50 Gy led to >50% tumoral volume reduction in a subcutaneous glioma C6-bearing rat model compared to the group treated with microvesicles at a size of 395.0 ± 202.6 nm from cells without irradiation. Apoptotic tumor cells in the irradiated microvesicle-treated group were three times higher than in the control group, thus the predominant antitumor mechanism of this irradiated microvesicle-based vaccine was attributed to apoptosis of tumor cells.316
In addition to the above external radiation-participated vaccination methods, injection of therapeutic radioisotope-incorporated nanomedicines is another practical and feasible approach to induce in situ vaccination. Clinically available 131I was selected as an internal radiation source, and alginate (ALG), a natural polysaccharide as an endogenous Ca2+-responsive gelation and fixed agent, was chosen to locally hold 131I-labelled catalase (CAT) and an immune-adjuvant CpG oligonucleotide at the tumor site. This “vaccine-alike” 131I-CAT/CpG/ALG displayed excellent immune memory protection with no apparent secondary tumor growth. No animal death was observed until the end observation time point at day 90. The percentage of effector memory T cells (CD3+CD8+CD62L−CD44+ T cells) in the spleen and the level of cytokines (TNF-α and TNF-γ) in the serum were significantly higher in CT26 tumor models treated with 131I-CAT/CpG/ALG than those treated with surgery and 131I-CAT/ALG.317 In another study, 131I-MnO2-BSA was systemically administered to CT26 murine colorectal tumor models. Compared with other therapies (control, MnO2-BSA, and free 131I), this radionuclide-based nanoformulation contributed to a 1–3-fold increase in matured DCs and a 1–2-fold increase in CD3+CD8+ cytotoxic T cells at the tumor sites.318
Overall, there remain a few challenges in this vaccination-mediated treatment. There may be a potential risk for in situ vaccination: burst release of tumor-associated antigens that are also expressed in non-tumoral tissues may result in nonspecific enhanced immune response to healthy tissues. Exogenous nanovaccination involves multiple procedures including loading tumor neoantigens, transporting to lymph nodes, maturing DC, and activating cytotoxic T lymphocytes. It is challenging for an exogeneous nanovaccine to be effective in these multiple procedures. Thus, cancer nanovaccines usually have insufficient vaccine potency for tumor eradication, and they are often accompanied by other therapies, including ICIs or chemotherapeutic drugs.
4.3 Prompting immunogenic cell death (ICD)
ICD is referred to cell death that elicits an immune response by recruiting and activating DCs to antigens released from dead or dying tumor cells.319,320 ICD is often induced after physical/chemical stresses on the endoplasmic reticulum and it is featured with a high production level of ROS and an apoptotic cell morphology that maintains membrane integrity.321,322 ICD inducers either prompt tumor cell death or induce the release of DAMPs. They are normally divided into two main types: type I acts as an ROS generator; and type II exerts escalated collateral stresses on the endoplasmic reticulum.323 Besides, the hallmarks of ICD are exposure of calreticulin (CRT) and heat shock proteins on the cell surface, active release of high mobility group protein B1 (HMGB1) and ATP, and late-stage presentation of uric acid.324,325 ATP provides a “find me” signal to recruit DCs. The DC phagocytoses the generated ERp57/CRT complexes to offer an “eat me” signal through their interaction with CD91, a DC receptor, and the NF-κB pathway to produce IL-6 and TNF-α. Notably, ICD is also strongly correlated with cancer vaccination.326,327Among ICD inducers from a physical energy source, such as a magnetic field,328 ultrasound,329 and near-infrared light,330 RT is the most accessible, acceptable, and well-studied therapeutic modality in clinics. When immunotherapy is introduced simultaneously, RT outperforms other inducers. Specifically, RT holds the following advantages: (a) deep tumor penetration; (b) high power energy;331 (c) localized therapeutic radioisotopes for internal radioisotope therapy that can mimic an irradiation field to reduce damage to normal tissues;332 (d) imaging-guided precise therapy via one integral instrument (IGRT or MR-Linac);333,334 (f) tunable biological effects induced at different doses;21,22 and (g) routine use without sensitizers.
In the context of radio-immunotherapy, RT induces upregulated expression of tumor antigens and inducible or constitutive DAMPs,335 but it generates too weak an effect to elicit sufficient ICD due to several endogenous resistance mechanisms and poor radiation absorption in some of tumor tissues (e.g., sarcoma).336 Insufficient ICD or low immunogenicity is also often seen in conventional ICI-based immunotherapy.337 The use of nanomedicines can boost the effect of ICD in radio-immunotherapy by either delivering adequate immune adjuvants to elicit strong immune response or escalating the antitumor effect of radiation, thus enhancing the therapeutic efficacies of immunotherapy. Cisplatin-loaded poly(L-glutamic acid)-graft-methoxy poly(ethylene glycol) complex nanoparticles (CDDP-NPs) were reported to significantly enhance RT-induced ICD. The percentage of CRT-positive Lewis lung cancer cells increased from 16.47% by treatment with RT to 27.03% after treatment with RT + CDDP-NPs, in comparison with 20.53% in the group treated with RT + cisplatin.338
A few nanosized ICD inducers, including nanoscale coordination polymers, high Z radiosensitizers, and natural polysaccharides, have been developed in combination with strategies of amplifying oxidative stress to induce a boosted ICD effect.
Nanoscale coordination polymers (NCPs) in which ROS-generating metal ions (e.g., Hf, Gd, Cu, or Bi) are incorporated into functional ligands or biomacromolecules (CpG oligodeoxynucleotides) have been reported to induce potent ICD via an elevated ER stress.262,339 For instance, in a recent study, gadolinium and zoledronic acid self-assembled to form a nanorod-like NCP, ZGd-NRs, with a long and short diameter of 200 and 20 nm, respectively. Evaluation of intracellular ROS and γ-H2Aχ in CT26 cells indicated that ZGd-NRs could efficiently amplify ROS generation and enhance X-ray deposition. The incorporated Gd3+ ions accounted for most of the radio-sensitization effect. The CT26 cells treated with ZGd-NRs and RT displayed a 1–4-fold increase in the ICD induction indicators including the level of CRT, HMGB1, and ATP in comparison with other three groups treated with saline, ZGd-NRs, and saline + RT. When the treatment with ZGd-NRs and RT was combined with αPD-L1, the infiltrated CD4+ and CD8+ T cells in both primary and distant tumors were nearly doubled. Furthermore, the functional ligand, zoledronic acid, helped in depleting TAMs to reprogram an immunosuppressive TME by causing apoptosis of macrophages (Fig. 9a).340 Another strategy to boost potent ICD was recently proposed. Mixed-valence copper (Cu+/Cu2+) was chosen as a metal ion candidate. 5′-Guanosine monophosphate (5′-GMP), widely presented in the body, acted as a coordination ligand for this Cu-NCP. Amplified oxidase stress was attributed to a synergistic effect via Cu+-mediated ROS generation and Cu2+-triggered GSH elimination. This nanomedicine in combination with RT increased CD8+ cell infiltration and IFN-γ expression in a 4T1 murine breast model. Furthermore, in a 4T1 primary and metastatic tumor model, the addition of αPD-L1 showed a better antitumor effect and a greater level of biosafety than other control groups, as evidenced by the primary tumor volume, the mice body weight, counts of lung metastatic sites, and the survival percentage (Fig. 9b).341 Similarly, gadolinium, 5′-GMP, and hemin (a peroxidase mimic), self-assembled to form H@Gd-NCPs. Hemin has a similar function to Cu2+, utilizing overproduced H2O2 to deplete GSH via peroxidase-mimic catalytic activity.261 To conclude, NCPs offer a versatile scaffold or model carrier for a vast library of ICD-inducing ligands, metal ions and immune-activation agents.
 | ||
| Fig. 9 Nanoscale coordination polymer-based ICD inducers for improving antitumor therapeutic outcomes. (a) Zoledronic acid–gadolinium coordination polymer nanorods (ZGd-NRs) aided in improving radio-immunotherapy: (i) scheme of the preparation procedure and TEM images of ZGd-NRs; three typical ICD biomarkers in CT26 tumor cells, (ii) CRT, (iii) HMGB1, and (iv) ATP, upon four different treatments (saline, ZGd-NRs, saline + RT, ZGd-NRs + RT). Reproduced with permission. Ref. 340 Copyright 2021, American Chemical Society. (b) Mixed-valence copper-based nanoscale coordination polymers (Cu-NCPs) for augmenting radio-immunotherapy: (i) preparation process and TEM images of Cu-NCPs; (ii) synergistic mechanism of Cu-NCPs with RT to promote ICD; (iii) growth curves of distant tumors and (iv) counts of lung metastases sites in tumor-bearing murine models after various treatments including saline, Cu-NCPs + RT + αCD8a, αPD-L1 + RT, Cu-NCPs + RT, and Cu-NCPs + RT + αPD-L1. Reproduced with permission. Ref. 341 Copyright 2021, Wiley-VCH. | ||
High-Z element radiosensitizers contribute to an enhanced performance of RT-induced ICD. They generate ROS in an intracellular oxygen-independent manner, contributing to the amplification of the endoplasmic reticulum stress, a crucial inducer for ICD.342 A snowflake-like Au nanocarrier with RT-responsive degradability, S-AuNC, was constructed to load anti-PD-L1. Under X-ray irradiation, the high-Z gold-based nanostructure produced a high ROS level, which irreversibly disintegrated the structure to release the loaded anti-PD-L1. Among all the treatment groups on murine Tramp C1 prostate cancer cells, RT plus S-AuNC induced sufficient ICD with a HMGB1+CRT+ cell population of 51.4%, in comparison with 37.4% in the group treated with RT. Additionally, the PD-L1 expression in the RT + S-AuNC-treated group was significantly increased by nearly 4 fold compared to that in the RT group, facilitating the following therapy with released anti-PD-L1 from the nanomedicine.343 Similarly, a heterojunction WO2.9–WSe2–PEG nanostructure (WSP NP) with high photocatalytic activity was developed to boost the intracellular ROS level, which could induce ICD. It was noticed that the high photocatalytic properties of this heterojunction structure were ascribed to the inherent properties of the high-Z element and the heterojunction structure because this structure was able to prevent the recombination of electrons and holes. The fluorescence intensity of ROS generated in the group treated with WSP NPs and X-rays was strengthened by 1.5 times compared to that in the X-ray-treated group. Confocal imaging and flow cytometry also confirmed sufficient induction of an ICD biomarker, CRT, in 4T1 cells treated with WSP NPs and X-rays.142 To conclude, high-Z nano-radiosensitizers can be used to prepare potent nanoformulations for promoting induction of ICD through increasing the ROS-mediated endoplasmic reticulum stress under low-dose external irradiation. Moreover, these nano-radiosensitizers in combination with ICIs could significantly improve antitumor immune response.
Natural polysaccharides including chitosan, hyaluronic acid, dextran, alginate, and Ganoderma lucidum polysaccharides have been reported to be immunoactive and promote DC maturation, a critical immunogenic indicator of ICD.98,344 For example, sulfhydryl-modified Ganoderma lucidum polysaccharides (GLP) were covalently conjugated to bismuth sulfide nanoparticles (BiNPs) to prepare GLP-BiNPs. BiNPs were synthesized via a BSA-mediated template approach. DC maturation was then investigated in the spleen/tumor of 4T1 breast tumor-bearing mice treated with a negative control, BiNP, and GLP-BiNP. In contrast to groups treated with BiNP and the control, the percentages of mature DCs in the tumor/spleen at a post-injection time point of 24 h or 72 h were higher in the mice group receiving GLP-BiNP. In the group treated with 4 Gy X-ray radiation at 4 h after intravenous injection of GLP-BiNP, the tumor volume was effectively reduced compared to other groups treated with a negative control, GLP-BiNP, X-rays, and BiNP + X-rays. The percentage of CD4+/CD8+ T cells in splenocytes and the concentration of IFN-γ and IL-4 in the group treated with GLP-BiNP + X-rays were significantly higher than those in other treatment groups.345 In another study, the Astragalus membranaceus polysaccharide was reported to induce DC activation through the TLR4 signaling pathway.195 Since the potential bio-effect induced by these immunoactive agents is very promising, their biological mechanism and biosafety should be examined in great detail, such as the triggered signaling pathways for inducing immune activation and the intensity, durability, and universality of induced immune activation.
Overall, the approaches to using NCPs, radiosensitizers, or natural polysaccharides to escalate the tumor ROS level and induce ICD-related immune response have been demonstrated to be able to induce potent ICD. At present, RT-induced ICD is vastly studied in cancer radio-immunotherapy, while a few studies focus on the potential role of mono-immunomodulator agents as the ICD inducer.
4.4 Overcoming resistance to radio-immunotherapy
Biological or pathological barriers, insensitivity to radiation therapy, and immunosuppression are three major resistances to cancer radio-immunotherapy. These three resistances are tightly interwoven due to their common drivers, such as hypoxia and myeloid-derived suppressor cells. As a result, biological or pathological barriers are addressed to mitigate the resistance to radio-immunotherapy in most cases, and mitigation in the radiation resistance may be accompanied by the effect of reversing immunosuppression, and vice versa.To address the physiological barriers, active targeting moieties are often incorporated or conjugated onto nanomedicines for precision therapy (Fig. 10a). Antibodies/nanobodies (e.g., anti-epidermal growth factor receptor and anti-human epidermal growth factor receptor-2), peptides (e.g., intercellular adhesion molecule-1 peptide, arginine–glycine–aspartic acid peptide, Cys–Arg–Glu–Lys–Ala peptide, and twin arginine translocation peptide), small molecules (e.g., folic acid, biotin, mannose, galactose, and sialic acid), and other active targeting ligands (e.g., epithelial cell adhesion molecule aptamer, dibenzocyclooctyne, and hyaluronic acid) have been used for improving accumulation and retention of nanomedicines in the region of interest. This strategy is well summarized elsewhere.350,351 To cross the brain–blood barriers, recently, an adenosine 2A receptor agonist has been reported to affect both F-actin and tight junctions of endothelial cells, and it could be a potential immunotherapeutic candidate for active targeting of brain malignancies.352 Similarly, an ApoE-peptide-modified polymersome was shown to successfully penetrate the blood–brain barrier in a mimicking in vitro model, a bEnd.3 murine endothelial cell monolayer. Furthermore, its immunoregulatory cargos (granzyme B and CpG) contributed to a prolonged survival duration in orthotopic glioma-bearing mice.353 In addition, an in vivo click chemistry-mediated approach was reported by harnessing the interaction between pre-azide group-modified lymphatic endothelial cells and a dibenzocyclooctyhne-functionalized cancer nanovaccine to increase the accumulation of the nanovaccine in the lymph node.354 Furthermore, pre-occupying the sites in the liver reticuloendothelial system is another approach to improving active targeting and bioavailability of drugs.355 In summary, these active targeting strategies, such as cancer nanovaccines targeting lymph nodes and radiosensitizers or nano-immunomodulators targeting the TME,356–358 could enhance the accumulation of the nanomedicines to exert their biological effects.
Besides RT-aided blood flow improvement, strategies of using nanomedicines for vascular normalization and ECM normalization are conducive to addressing the disease-specific barriers.359,360 Vascular normalization could be achieved by utilizing antiangiogenic agents (e.g., anti-VEGF and nitric oxide-based prodrugs) or other therapeutic interventions (e.g., low-dose radiation) to address issues originating from abnormal tumor vasculature, allowing adequate perfusion of medicinal drugs and tumor oxygenation (Fig. 10b).361 These therapeutic benefits are often dose- and time-dependent.362 It was reported that blocking the insulin-like growth factor-binding protein 7 (IGFBP7)/CD93 axis using mAbs in two mice models, an orthotopic KPC tumor model and a B16 tumor model, contributed to tumor vascular normalization, which in turn sensitized tumor cells to ICI therapy.363 Besides, folic acid-incorporated gold nanoparticles (AuNPP-FA), composed of folate, a PEG block, a PDEAEA block with tertiary amines, and stabilized gold nanoparticles, were applied to normalize the tumor vascular structure through strengthening tight junctions by upregulating the VE-cadherin level and increasing the pericyte coverage. Notably, a correlation between tumor vascular normalization realized by AuNPP-FA and tumor metastasis inhibition was established, indirectly supporting the potential effect of vascular normalization on reducing metastasis.364 In another report, imidazole-incorporated organosilica nanochelators with a copper/phosphate responsive aggregation behavior were developed to realize antiangiogenesis and vascular obstruction after depleting Cu2+. An elevated level of Cu2+ and phosphate in the tumor environment was significantly reduced because the coordination interaction between imidazole or phosphate and Cu2+ led to aggregation of these dispersed sub-6 nm nanochelators. A significant antitumor effect was achieved in both 4T1 breast cancer and CT26 murine colon cancer models after treatment with nanochelators compared to other groups treated with saline or a copper chelator TM in the clinical trial.365
ECM normalization regulates the dense, aberrant tumor ECM and vessel compression, enabling homogenous distribution and enhanced penetration of nanomedicines (Fig. 10c). Abundant cancer-associated fibroblasts (CAFs), collagen I, and hyaluronan may be the main therapeutic targets. CAF, a driver for fibrosis generation, matrix remodeling and immune crosstalk, may exert negative effects on radio-immunotherapy and hence become a leading therapeutic target.366 Specific targets and inhibitors for CAFs have been discovered. Cyclopamine, a sonic hedgehog inhibitor, was reported to deplete stroma-producing CAFs in a polymeric micelle nanoformulation.367 Fibroblast activation protein (FAP) that is overexpressed in CAFs was reported to be successfully targeted by nanomedicines modified with a quinoline-based FAP-inhibitor variant (FAPI-46).368,369 Moreover, SLC7A11, a cystine transporter in pancreatic ductal adenocarcinoma (PDAC) tumor stroma-derived CAF, was identified to be independently prognostic of poor overall survival. A gene-silencing nanomedicine against SLC7A11 was thereafter developed and proved to be able to reduce tumor growth, CAF activation, and fibrosis in orthotopic murine PDAC models.370 For stroma-rich collagen and hyaluronan, a significant decrease in ECM levels (e.g., collagen) in 4T1 metastases nodules was recently observed after the treatment of tranilast and liposomal Doxil. Specifically, the fraction areas of collagen I, hyaluronan, and hypoxia in this treatment group were significantly reduced, while the density of the perfused vessels increased in contrast to other treatment groups. As a result, this nanomedicine-induced vessel decompression effect resulted in an elevated antitumor effect of ICI in two immunotherapy-insensitive metastatic breast cancer models (4T1 and E0771).371 In addition, hyperbaric oxygen (HBO) therapy could leverage high-pressure oxygen (2.5 atm) to overcome stroma-rich solid tumors, including PDAC, hepatocellular carcinoma, and triple-negative breast cancer, through depleting main components of the extracellular matrix (collagen) via multiple mechanisms, such as downregulating the gene of TGF-β as well as the genes related to the CXCL12/CXCR4 signaling axis and disrupting hypoxia-mediated immunosuppression. Thus, this physical therapy may be a potential addition to current nanomedicine-assisted radio-immunotherapy.372
Hypoxia is the primary factor that leads to radiotherapy insensitivity or radiation resistance because the indirect DNA-damaging effect of RT predominantly relies on oxygen in the TME.374 Hence, improving tumor oxygenation or enhancing oxygen-independent free radical generation are two promising approaches for relieving hypoxia-induced radioresistance in the TME. Improving tumor oxygenation can be realized by increasing intratumoral blood flow, delivering or generating oxygen, and reducing the expression of hypoxia-inducible factors (e.g., HIF-1α) in the TME.375 The use of a high-Z radiosensitizer for selective RT irradiation on hypoxia tumor regions or a nano-catalyzer (e.g., catalase and holo-lactoferrin) contributes to oxygen-independent free radical generation.376,377
Exogenous oxygen carriers, for instance, hemoglobin (Hb) and perfluorocarbon (PFC), as well as in situ oxygen generators, like the combined use of H2O2 and catalase, have been employed for tumor re-oxygenation to relieve hypoxia for radio-immunotherapy.115 PFC@lipo, Hb@lipo, and PX-478, a HIF-1α inhibitor, were investigated on their hypoxia relief performance. Hb@lipo treatment was found to be the best among these three candidates because of its more moderate oxygen release, a higher level of tumor accumulation through systemic administration, and a safer profile compared to the other two treatments.118 In another study, Hb, a high-Z radiosensitizer (hafnium, Hf), gallic acid, a natural metal chelator, and catechol/Ce6-modified 8-arm PEG, self-assembled into Hb@Hf-Ce6 nanoparticles. The fluorescence intensity of the generated 1O2 by Hb@Hf-Ce6 nanoparticles under X-ray irradiation (8 Gy) was 7.29 times higher than that of free Ce6, indicating this combined strategy achieved a synergistic ROS-generating effect. Furthermore, treatment with Hb@Hf-Ce6 + RT + anti-PD-1 in a bilateral 4T1 murine breast tumor model and its lung metastatic model exhibited remarkable control of tumor progression and an apparent reduction in lung metastasis.378 Moreover, a polydopamine-NP-stabilized oxygen microcapsule was also investigated as a new oxygen carrier to alleviate tumor hypoxia. Oxygen was sheared into microbubbles, and dopamine was then oxidized in an alkaline environment. Amino-rich polylysine and chitosan were used to aid in accelerating the interfacial polymerization of dopamine nanoparticles. Finally, glutaraldehyde was used for the permanent cross-linking of these NPs. Immunohistochemistry results showed that the expression of HIF-1α, an indirect indicator of the hypoxia degree, was much lower in the hep1–6 murine tumor mice group treated with local oxygen microcapsules and local oxygen microcapsules + RT (8 Gy × 2 fractions) in comparison with other groups, indicating that oxygen microcapsules could effectively relieve tumor hypoxia.379
Catalase (CAT), a nano-catalyzer and an in situ oxygen generator, can catalytically accelerate the decomposition of H2O2 into oxygen at the tumor site. External delivery of CAT and H2O2 into tumors can contribute to robust tumor oxygenation. For instance, CAT@liposome and H2O2@liposome were intravenously injected into 4T1 murine breast tumor-bearing mice in sequence at a 4 h interval. The hypoxia positive areas in ex vivo immunofluorescence staining images of tumors after different treatments were semi-quantitatively assessed. The percentage of hypoxia was 61% for the group treated with PBS and 63% for the group treated with H2O2@liposome, while it reduced to ∼27% and ∼9% for the group treated with CAT@liposome and CAT@liposome + subsequent H2O2@liposome, respectively. As a result, the tumor oxygenation method via CAT@liposome + subsequent H2O2@liposome significantly improved the antitumor effect after treatment with radio-immunotherapy (X-rays + anti-CTLA-4).380 A similar improvement in tumor re-oxygenation was observed in a PLGA-R837@CAT nanoparticle. In this nanoparticle, water-soluble CAT was encapsulated into the core of PLGA, and a TLR-7 agonist, imiquimod (R837), was loaded onto the shell of PLGA. Cancer radio-immunotherapy of a subcutaneous CT26 model and an orthotopic 4T1 metastasis model via X-rays + αCTLA4 resulted in a distinctive antitumor effect after application of PLGA-R837@CAT nanoparticles.381
Additionally, nitric oxide (NO), an exogenous regulating agent, aids in relieving tumor hypoxia by modulating angiogenesis. A few NO donors, including sodium nitroprusside,383S-nitrosothiols,384D-arginine,117L-arginine,385 DETA NONOate,386 and 3-morpholinosydnonimine hydrochloride,387 have been successfully used to prepare NO-generating nanomedicines to alleviate tumor hypoxia. A NO-generating micelle was formed via self-assembly through thin-film hydration from D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS)-NO. Under a high GSH tumor environment,388,389 this micelle could release NO in a reductive-responsive manner. In vivo PET-CT imaging with a hypoxia-specific probe (18F-MISO) confirmed that TPGS-NO and TPGS-NO + RT could effectively reduce tumor hypoxia regions in LLC tumor-bearing mice. Consistently, immunofluorescence imaging and western blotting analysis demonstrated a significant decline in HIF-1α and Glut1 expression in tumor tissues after i.v. injection of TPGS-NO (50 mg kg−1) every two days in the first week and RT (8 Gy) on day 5. Consequently, this TPGS-NO + RT approach contributed to a pronounced antitumor effect over other groups treated with PBS, TPGS-NO, and RT.390D-Arginine, another source of NO, was loaded onto the surface of hyaluronic acid-modified MIL-100 (Fe) to form HA@MOF/D-Arg, which was used to improve the radiosensitivity of K7M2 murine osteosarcoma. It was shown that by down-regulating HIF-1α expression, tumor hypoxia was alleviated in the mice group treated with HA@MOF/D-Arg + X-rays. More importantly, the lung metastasis sites of osteosarcoma were found to be significantly reduced compared to other treatment groups including PBS + X-rays, D-Arg + X-rays, and HA@MOF + X-rays. In addition, this combined therapy demonstrated an encouraging tumoricidal effect over other therapies. The control of tumor growth was found to be in a descending order of HA@MOF + X-rays, D-Arg + X-rays, and PBS + X-rays (Fig. 11a).117 In another report, a 32P-labelled single-layer 2D nanosheet, ZnNO(32P), was fabricated using Zn ions, sodium nitroprusside (Na2Fe(CN)5NO) as a NO donor, and 32P radioisotopes. In their design, the therapeutic radioisotope 32P with β-ray emission efficiently activated water to generate strong Cerenkov luminescence (CL) and subsequently triggered NO release. Radioisotope tracing and CL intensity monitoring in the tumor suggested that the tumoral retention time of intratumorally-injected ZnNO(32P) was slightly longer than that of ZnCN(32P) but much longer than that of free 32P. Meanwhile, this sustainable NO-releasing strategy significantly improved the hypoxia-mediated immunosuppressive tumor environment. The immunofluorescence images of tumor slices from different mice groups confirmed that the tumors treated with ZnNO(32P) exhibited the smallest HIF-1α and Treg positive areas and the highest CTL positive area or the highest CTL/Treg ratio. Furthermore, the combinational treatment with ZnNO(32P) and anti-PD-1 elicited a potent and durable immune response. The CTL positive percentages in local and distant tumors in a CT26 colon tumor model were the highest after this combinational treatment. Meanwhile, the concentrations of immunomodulators, including IFN-γ and TNF-α, continuously increased and they surpassed those in other groups including PBS, anti-PD-1, and ZnNO(32P) on day 0, day 7, and day 12 (Fig. 11b).382
 | ||
| Fig. 11 NO-generating MOFs for enhancing radio-immunotherapy through sensitizing the TME. (a) D-Arginine-loaded MOFs increased the radio-sensitivity of osteosarcoma: (i) illustration of the synthetic route for HA@MOF/D-Arg; (ii) scheme of the radio-sensitization mechanism via HA@MOF/D-Arg; (iii) free radicals and (iv) NO gas generated from HA@MOF/D-Arg, which were measured from the intensity of their specific in vitro probes; (v) antitumor effects of the combination therapy of HA@MOF/D-Arg with X-ray RT (indicated with arrows), PBS + X-ray, D-Arg + X-ray, and HA@MOF + X-ray were used as controls. Reproduced with permission. Ref. 117 Copyright 2021, Elsevier. (b) 32P-Labelled ZnFe(CN)5NO nanosheets aided in improving radioisotope-immunotherapy: (i) illustration of the lattice structure, cerenkov luminescence-induced NO release, and TME priming effect of 32P-labelled ZnFe(CN)5NO nanosheets; (ii) immunofluorescence images of tumor slices after various treatments. Blue DAPI for cell nucleus, and green HIF-1α for hypoxia; (iii) released NO concentrations from ZnNO(32P), ZnNO, and free 32P at different incubation time points; quantitative analysis of (iv) HIF-1α positive area and (v) CTL/Treg ratio in tumor slices resected from murine tumor models at 12 h post-treatment with various agents, including PBS, ZnNO, free 32P, ZnCn(32P), and ZnNO(32P). Reproduced with permission. Ref. 382 Copyright 2019, Elsevier. | ||
Overall, nanomedicines have been developed for tackling tumor hypoxia and other radio-resistance factors to precisely deliver/generate oxygen, nitric oxide, HIF-1α inhibitors, radiosensitizers, or other similar functional agents in the tumor hypoxia region. Besides, strategies to overcome radioresistance of tumors have been formulated. For example, dual blockage of RT-induced upregulated CD47 and HER2 eliminates radioresistant HER2+ breast cancer.391 However, challenges remain to be addressed, including rapid diffusion and clearance of gas (oxygen and nitric oxide), a low amount of gas generated from these nanomedicines, and delivery of these nanomedicines to the tumor hypoxia regions that are often located far from blood vessels.
Inhibiting TAMs, MDSCs, or Tregs. TAMs, MDSCs, and Tregs are critical drivers for immunosuppression in tumors.398 TAMs include pro-tumoral M2-like macrophages in a large population and anti-tumoral M1-like macrophages in a minor population.399 Depleting or reducing the recruitment of M2-like TAMs, MDSCs, or Tregs has been demonstrated to successfully reverse immunosuppression.400–403
TAM-depleting bisphosphonates, a clinically-used drug,404 were reacted with calcium ions via a reverse microemulsion method. The product was modified with PEG for labelling with diagnostic or therapeutic radioisotopes. Cationic 99mTc4+ was labelled onto this nanomedicine with a radiolabelling yield of 70% by coordination between technetium and phosphonate for single photon emission computed tomography imaging, while 32P, in the form of anionic 32PO43− as a pharmaceutic active ingredient, was labelled via anion exchange. This radiolabelled CaBP(99mTc4+)-PEG nanomedicine displayed a potent tumor-homing ability with a tumor uptake amount of 5.4% ID g−1 at 24 h post-injection. The F4/80-positive area for TAMs was significantly reduced to 0.23% after CaBP-PEG treatment in comparison with 10.74%, 11.3%, and 3.67% in the groups treated with PBS, CaP-PEG, and free BP, respectively. Similarly, the TAM-stimulated EGFR-positive area shrank to 35% in the CaBP-PEG-treated group in comparison with 67%, 64%, and 52% in the control groups treated with PBS, CaP-PEG, and free BP, respectively. Furthermore, the addition of therapeutic radioisotope 32P to CaBP-PEG NPs showed an excellent synergistic antitumor effect with a combination therapeutic index of 0.48. These results confirmed that CaBP-PEG NPs could effectively deplete TAMs and offer a favorable tumor microenvironment for the following radiotherapy (Fig. 12a).405
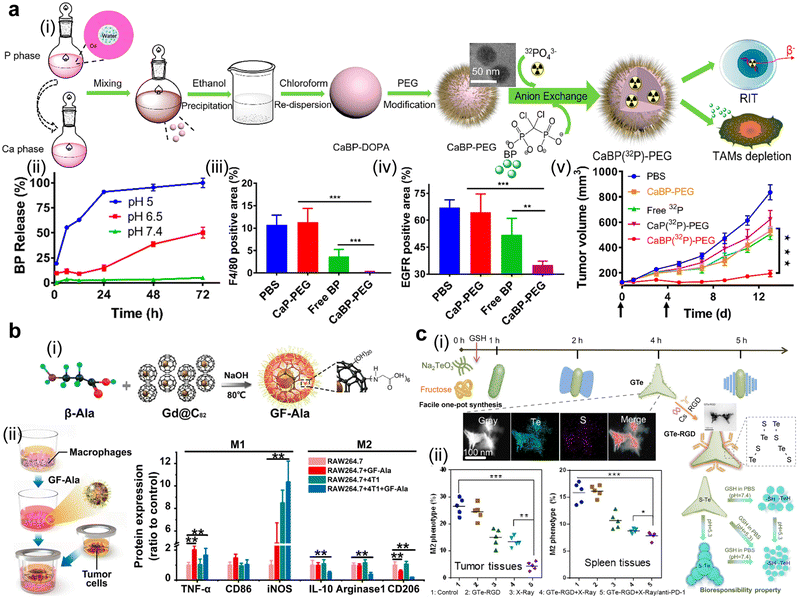 | ||
| Fig. 12 Nanomedicine-assisted approaches to eliminating or polarizing TAMs. (a) Chelator-free, radionuclides-labelled calcium bisphosphonate enhanced cancer radiotherapy through depleting TAMs: (i) illustration of the preparation procedure of radio-labelled CaBP-PEG nanoparticles; (ii) release of bisphosphonate (BP), a macrophage-depleting agent, from this NP under different pH conditions; quantitative analysis of (iii) F4/80-positive and (iv) EGFR-positive areas in tumor slices from a murine tumor model after different treatments; (v) tumor growth curves of different treatment groups upon administration two times indicated with arrows below the horizontal axis. Reproduced with permission. Ref. 405 Copyright 2018, American Chemical Society. (b). Gadofullerene (GF-Ala) nanoparticles repolarized TAMs into M1-type ones: (i) scheme of the synthetic route for GF-Ala; (ii) the flow chart of a co-culture cell system and quantitative analysis of M1 or M2 markers-associated protein expression after different incubation conditions. Reproduced with permission. Ref. 172 Copyright 2020, American Chemical Society. (c) Triangle-shaped tellurium nanostars enabled TAM repolarization to potentiate radio-immunotherapy: (i) illustration of the preparation procedure of GTe at different reaction durations, as well as its biological function; (ii) the percentage of M2-type TAMs in the tumor and spleen tissue under different treatments. Reproduced with permission. Ref. 406 Copyright 2020, Elsevier. | ||
In an attempt to deplete MDSCs, chitosan/poly(γ-glutamic acid) nanoparticles, Ch/γ-PGA NPs, were developed as a functional adjuvant to RT. RT was used to reduce the primary tumor burden, while Ch/γ-PGA NPs were applied to decrease splenomegaly and lung metastases and relieve systemic immunosuppression. Consequently, the percentage of immunosuppressive myeloid cells (CD45+CD11b+) in the spleen and the weight of the spleen in the 4T1 tumor-bearing mice group were reduced.407 In a murine model of GL261 glioma, the PD-L1 level in tumor-associated myeloid cells (TAMCs), including M-MDSCs, PMN-MDSCs, and TAMs, was found to be the highest compared to other glioma-infiltrating immune cells (microglia, DC, CD8+ CTL, CD4+Teff, and Tregs). Based on this finding, a TAMC-targeting lipid-based nanoscale system with surface-modification by αPD-L1 and encapsulation of dinaciclib (a cyclin-dependent kinase 5 inhibitor) was developed to treat TAMCs. Specifically, flow cytometric analysis confirmed that via the Rhod-PE-labelled approach, αPD-L1-LNP exhibited a high efficiency in targeting in vitro-generated TAMCs, as well as human-derived TAMCs, which were isolated from the peripheral blood and tumor tissue of glioblastoma patients. In addition, the viabilities and immunosuppressive activities of TAMCs were effectively impaired by this αPD-L1-LNP. Furthermore, thanks to the effective scavenging of TAMCs to prevent an increase in their PD-L1 level after RT, the combination of RT with αPD-L1-LNP effectively extended the survival time of two syngeneic gliomas models of GL261 and CT2A compared to that treated with RT.408
Polarizing tumor-associated macrophages. Polarizing pro-tumor M2-like TAMs to the pro-inflammatory ones is another promising approach to overcoming immunosuppression. Immunomodulatory agents, such as NO donor S-nitrosothiol, anti-CD40, toll-like receptor agonists (resiquimod or imiquimod), bexarotene, chlorogenic acid, and TMP195, have been reported to aid in this transformation process.409–412 For instance, it has been reported that lipidic polyplex-aided delivery of the plasmid of IL12, a pro-inflammatory chemokine, could increase the ratio of M1/M2 by more than four times.413 Moreover, Ginseng-derived nanoparticles (GDNPs), isolated from Panax ginseng C.A. Mey, were found to polarize TAMs to the M1-like one via the TLR-4/myeloid differentiation antigen 88 signaling pathway.414
Specifically, β-alanine-modified Gd@C82 (GF-Ala) nanoparticles were reported to re-educate TAMs to a tumoricidal M1-like phenotype through the activation of NF-κB and IRF5 pathways. This fabricated GF-Ala nanomedicine had an average diameter of 68.1 ± 2.3 nm and a negative surface potential of −37.7 ± 0.3 mV. After treatment of RAW264.7 cells with the nanomedicine, the expression of TNF-α, a classical M1 marker, was sharply increased over 3.6 times than that in the untreated RAW264.7 cells. Meanwhile, the level of IL-10, a classic M2-related maker, was 32.6% lower in the GF-Ala-treated group than that in control cells without any treatment. The nanomedicine in combination with anti-PD-L1 to treat a 4T1 tumor model exhibited a pronounced synergistic antitumor effect. Given the radio-sensitization properties of high-Z gadolinium, its antitumor effect could be further improved with the intervention of RT (Fig. 12b).172 In another report, a metallic radiosensitizer-assisted radio-immunotherapy (external RT + anti-PD-1) was developed and effectively reduced the percentage of M2-like macrophages. L-Glutathione-modified triangle-shaped tellurium nanostars were prepared via a shape-control one-pot hydrothermal method. Their following surface-decoration with a targeting ligand, RGD (Arg–Gly–Asp), and biocompatible chitosan constituted a radiation-driven immunotherapeutic enhancer, GTe-RGD. In vitro experiments demonstrated that GTe-RGD could improve the radiation sensitization of tumor cells including A375, HeLa, and 4T1 cells. GTe-RGD exhibited GSH-responsive degradability, promoting its in vivo clearance after its therapeutic function. Furthermore, the percentage of the M2 phenotype was much lower in the tumor tissue and slightly lower in the spleen tissue in the 4T1 breast murine mice group treated with GTe-RGD + X-rays/anti-PD-1 than that in the groups receiving PBS, GTe-RGD, X-rays, and GTe-RGD + X-rays (Fig. 12c).406 Additionally, the joint use of hydrophobic imiquimod and hydrophilic αCD47 co-delivered by an Hf-DBP nMOF exerted a TAM repolarization effect in bilateral CT26 tumor models. In this study, imiquimod, a TLR-7 agonist, was loaded in the pore of the MOF and αCD47 was attached onto the surface of the Hf-DBP nMOF with acetate capping groups. In contrast to the PBS-treated group, this nanomedicine-mediated treatment increased the M1 population by 7.6% and reduced the M2 population by 15.7%. When the nanomedicine was further combined with RT, a nearly one-fold increase and drop was observed in the percentage of M1 and M2 cells, respectively.415
Disturbing immunosuppression-related pathways and immunosuppressive agents. In cancer sites, such as liver metastasis sites, TAMs, MDSCs, Kupffer cells, and hepatic dendritic cells collectively form and support a network of active immunosuppressive pathways, suppressing the activation of CD8+ effector and CD4+ T cells and inducing immune tolerance to immunotherapy.396,416,417 In addition, an increase in the level of TNFα and TGF-β induced by RT, and the rapid formation of adenosine from ATP released by RT could be the culprits of immunosuppressive agent-mediated immune tolerance.418–420 In detail, RT-induced TGF-β exerts multiple immune-suppressive activities, such as suppressing effector T cells, promoting tumoral infiltration of Tregs, and driving DCs to the immune-tolerogenic phenotypes.421 To conclude, these immunosuppression-related signaling pathways and immunosuppressive agents could be harnessed to overcome resistance to immuno-oncology-associated treatments.
Specifically, it has been reported that the inhibition of the BTK-PI3Kγ signaling pathway and blocking the CD47-SIRPα axis contribute to reverse immunosuppression.422 Furthermore, the blockade of the CCL2/CCR2, CXCR4-CXCL12, CSF-1/CSF-1R signaling pathways effectively prevented macrophage infiltration in the tumor, and the inhibition of CSF-1R in macrophages promoted therapeutic antibody-dependent phagocytosis of cancer cells.423–426 For example, a synthetic protein nanoparticle, comprising human serum albumin, fluorescently-labelled bovine serum albumin, iRGD, AMD3100 (a CXCR4 antagonist), and bifunctional macromer, has been shown to block the CXCR4-CXCL12 pathway in the orthotopic genetically engineered glioblastoma murine models. Furthermore, its combination with RT (2 Gy × 10 fractions) promoted an extended survival time. Three mice survived over 60 days, while all the mice in other treated groups were dead.426
Overall, despite a small number of studies on the use of nanomedicines to intervene immunosuppression-related signaling pathways and immunosuppressive agents,420,426 inhibitors for these pathways and agents can be incorporated into nanocarriers for overcoming immunosuppression, improving their solubility and bioavailability while reducing their toxicity induced from their accumulation in the normal tissue.
4.5 Remission of the therapy-induced toxicity issue
Nonspecific deposition of high radiation energy and distribution of immuno-oncology agents during cancer radio-immunotherapy are the primary toxicity-inducing factors. RT-induced acute and late-onset adverse events include brain damage (edema, radionecrosis, neurocognitive impairment, and brain inflammation), lung injury (pneumonitis and fibrogenesis), and lymphotoxicity.427 These adverse events have been reported to be associated with the cGAS-STING signaling pathway,428 while the toxicities from the immuno-oncology treatment depend on immunotherapeutic drugs. The use of ICIs is often accompanied by multi-organ inflammation (derma, gastrointestinal, and endocrine),429,430 while CAR T cell therapy and cytokine therapy may lead to the cytokine release syndrome and/or the immune effector cell-associated neurotoxicity syndrome.431From the above discussions, it can be seen that the adverse events induced by RT and immunotherapy are strongly related to inflammation. High-dose steroid therapy can effectively relieve the severity of inflammation. However, steroid-refractory irAEs, such as nonendocrine irAEs, are insensitive to this therapy.432 Therefore, protective drugs, detoxicant agents, or other strategies to reduce radio- or immuno-related toxicities have been explored. A couple of agents, including memantine, selenium, and anti-CD47 antibody, confer a radioprotective effect to the brain tissue undergoing whole-brain radiotherapy and the adjacent normal tissues predominantly via a ROS-scavenging manner.433–435 Meanwhile, masking agents or antibody-clearance agents are dedicated to relieving immune-associated toxicity.
Radiation proctopathy (RP) is the result of inflammation of the colorectal tissue after RT, in which the platelet-derived growth factor C (PDGF-C) and excessive ROS can act as therapeutic targets.436 In one study, upregulation of PDGF-C was found in the tissues with RP either from patients or animal models and genetic deletion of PDGF-C in mice could relieve RP-associated adverse effects. In their following experiments, the use of crenolanib, a selective agonist for PDGF receptors, in a PEG-400-based solubilizer, successfully prevented and/or ameliorated RP in mice, supported with a 20% thickness reduction of the submucosal layer and a decreased level of expression of fibrosis markers (α-SMA and fibronectin) in a mice model 8 weeks after RT.437 Another study employed spherical polydopamine nanoparticles (PDA-NPs) at 50 mg kg−1via an oral administration route to scavenge ROS and suppress inflammation in RT-induced intestinal injury murine models. Successfully, the content of MDA, an indicator of the lipid peroxidation of intestines, was reduced by ∼3 fold or ∼1 fold in RT + PDA-NPs on day 1 or day 3.5 post-radiation, respectively, compared to that with radiation only. Moreover, from immunohistochemical assays and fluorescence imaging, the PDA-NPs + RT-treated mice group exhibited superior protection of intestinal-associated cells (Lgr5+ intestinal stem cells, lysozyme+ Paneth cells, ki67+ crypt base columnar cells, or villus+ intestinal cells length) than the radiation-only group.266
The application of customized masking agents to develop immunotherapeutic prodrugs for switchable immune modulation is another example of using nanomedicines to realize tumor-specific activation of antitumor immune response and prevention of undesirable normal tissue accumulation.438 Engineering interleukin-based prodrugs is a preferable method to reduce immune-toxicity without compromising their therapeutic efficiency. For instance, an IL-2 mutein/Fc fusion protein, a biological binding site for CD8 T cells, was masked with an IL-2 receptor via a matrix metalloproteinase (MMP)-cleavable linker. Similarly, a recent study employed the masking strategy to reduce the immunotoxicity of IL-12. They fused a domain of the IL-12 receptor to IL-2 via a MMP-responsive linker.96 Therefore, the masking strategy via nano-engineering protease-sensitive linkers significantly reduces the toxicity in cytokine-based immunotherapy, meanwhile, the specific-site drug distribution and the antitumor effect have been greatly improved. In addition, a significant shortcoming of current antibody-based immunotherapy is their excessive blood circulation time, which may lead to the formation of thrombus and other adverse effects. These adverse effects and their immunogenic properties may cause damage to healthy tissues after the administration of these antibodies. Nanosized click antidotes, avidin chases, and other nano-agents have been employed for blood clearance of antibodies or nanomedicine-mediated strategies have emerged to accelerate blood clearance of these antibodies (Fig. 13).219,439–442
 | ||
| Fig. 13 Scheme for emerging nanomedicine-mediated strategies to accelerate blood clearance of antibodies. Various scavenging nano-agents have been designed to combine excessive engineered antibodies in the blood stream for rapid clearance via five main strategies, including tco-tetrazine, azides-cyclooctynes, complementary DNA, biotin–streptavidin, and bsAbs-haptens. Ref. 219 and 439–442. | ||
Overall, a few toxicity-inducing mechanisms of cancer radio-immunotherapy are well-studied, while the majority of them are still unknown. Improved biodistribution and pleiotropic immunomodulatory effects of nanomedicines, as well as employment of specific radio- or immune-protective drugs, can help in addressing issues of already identified toxicities. Non-specific body retention of nanomedicines should be reduced for their long-term safety. Renal- or hepato-clearance strategies have been applied to the design of these nanomedicines. Quantitative structure–activity relationships of the nanomedicines could be examined to reduce their toxicities. More importantly, toxicity from the common composition of nanomedicines should be seriously considered. Take PEG for example, the PEGylated approach can greatly alleviate the systemic toxicity of IL-2 (extensive inflammation and a fatal vascular leak syndrome), a cytokine used in adoptive cell transfer for T-cell expansion in clinical practice, which originates from non-specific distribution of IL-2 receptors.443 According to a recent report, PEGylated liposomes containing TLR agonists induced hypersensitivity reaction upon multiple dosing, which may be attributed to the generation of anti-PEG IgGs.444
5. Considerations for clinical translation and future directions
Clinical translation of nanomedicine-mediated radio-immunotherapy will benefit more cancer patients. However, numerous factors need to be carefully considered before these nanomedicines enter clinical trials. We will summarize the challenges in this field, discuss lessons learned from ongoing or completed clinical trials on radio-immunotherapy, and provide insights into challenges and future research direction of this combined therapeutic methods.445–4485.1 Summary of the challenging issues for radio-immunotherapy
In the past five years, a myriad of nanoformulations have been explored for radio-immunotherapy. This nanotechnology-aided therapy, mostly in the preclinical stage, helps in addressing a low response rate and systemic toxicity, as well as realizing therapeutic benefits, including effective systemic tumor control and long-term immune memory protection. However, challenges remain in the current trials of nanomedicines for radio-immunotherapy. Three prominent challenges are highlighted below.(i) Current nanoscale delivery systems are complex. Excess-engineering of the chemical structure and equipping with many functional moieties are often sought in the preparation of nanomedicines for realizing radio-immunotherapy. The primary concern of these nanomedicines is the reproducibility during their preparation process. Furthermore, the interaction between nanoformulations (e.g., PEGylated nanomedicines) and biological organs/tissues/cells still largely remains unknown.449 In particular, great efforts are needed to discover the interaction between these nanomedicines with a complex structure and the systemic immunity in the patient. More importantly, incorporation of multiple biological active agents in one single nanoplatform may increase the complexity of unveiling their synergistic mechanisms of action. Thorough investigations of a single promising active agent in the nanomedicine should be conducted to reveal its working mechanisms and additional active agents could be expanded to realize synergistic therapeutic benefits. In this context, MnO2 NPs may be a great candidate for radio-immunotherapy of cancer. MnO2 nanomedicine-mediated immunomodulation and radiosensitization have been discovered to some extent: Mn2+ augments the STING signaling and hypoxia-mediated radioresistance is relieved by oxygen generation in a MnO2-mimic catalyst decomposition of over-expressing H2O2 in TME.116
(ii) There are too many combinational treatment options. Many factors could play a role in the application of the nanomedicine as a mono-therapeutic agent for radio-immunotherapy or an adjuvant to RT and immunotherapy. These factors include administration routes, sequence, administration intervals, radiation doses, fractionation, the radiation source, and doses of the therapeutic agent(s) and the nanocarrier. Consequently, superfluous treatment plans could be formulated from the combination of the above factors, but these plans for a nanoformulation could not be fully executed considering the experimental cost and resources. It is advised to narrow down these factors based on previous and ongoing clinical trials and practice. In view of the complex radiation-immunity net, we suggest establishing a radiation dose and fractions for one indication. Robust immunity-eliciting radiation schemes (e.g., 8 Gy × 3 fractions) have been applied to compare the synergistic effects of various immunomodulatory nanoagents.52 Meanwhile, a hypofractionated low-dose radiation plan (e.g., 2 Gy × 5 fractions) is combined with one immune-drug, such as an ICI, to compare radiosensitivity and the synergistic abscopal effect of different metal or non-metal radiosensitizers.450,451 Additionally, the initial tumor volume, administration frequency, dosing, administration route, and therapeutic sequence could be standardized to establish the evaluation criteria for these nanomedicines.
(iii) Complete profiles of the immunogenicity and immunotoxicity of nanocarriers need to be established. The immunotoxicity of PEGylated liposomes used as a vaccine was recently revealed,452,453 including the anti-PEG IgG-induced hypersensitivity reaction.444 However, there are very few reports on thorough characterization of immunogenicity and immunotoxicity of polymer-, protein-, or inorganic-based nanocarriers. The standardized characterization methods should be established to accelerate the translation of nanomedicines for clinical trials. Recently, polysaccharides,454 cell membranes of treated tumor or immune cells,455,456 and bacterial outer membranes are pursued as personalized nanocarriers because they can elicit robust adaptive and innate immune response.457 The immunogenicity and immunotoxicity of these personalized nanocarriers are two of the most critical quality attributes (CQAs). The impurities in the preparation of these nanocarriers, such as endotoxin and chemical residuals, may also induce innate immune response, which could provide an inaccurate profile of immunogenicity and immunotoxicity of personalized nanocarriers.
5.2 Lessons learned from ongoing clinical trials
Over 500 clinical trials have been registered in the NCT database (https://clinicaltrial.gov/ct2/home) on cancer radio-immunotherapy during the past five years. Although there are a very few trials on nanomedicine-assisted radio-immunotherapy, the number of trials has been steadily increasing due to unique advantages and attributes of these nanomedicines as well as an increase in the approval of nanomedicines for other therapies. Strengths and weaknesses of these clinical trials could provide great insights into effective clinical translation of this nanomedicine-assisted combined therapy. Particularly, lessons from the the terminated, withdrawn, or suspended trials can help in designing and preparing nanomedicines, building patient cancer-mimicking animal models, and formulating treatment procedures for this radio immunotherapy. We have listed a few lessons from these trials.(i) Recruitment of inadequate patients for trials. A high risk of a new treatment may deter patients from participating these trials. Some of the enrollment criteria are set too high and very few patients can meet the enrollment criteria. A clinical trial screening tool to analyze 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 095 potential participants revealed that 13% of them did not enroll into the trial because they did not meet the trial eligibility.458 Solid preclinical data should be obtained to provide consistent and efficacious treatment in animal models. The clinical benefits of this treatment should be easily understood by these patients.
095 potential participants revealed that 13% of them did not enroll into the trial because they did not meet the trial eligibility.458 Solid preclinical data should be obtained to provide consistent and efficacious treatment in animal models. The clinical benefits of this treatment should be easily understood by these patients.
(ii) Poor or slow accrual. Many clinical trials are terminated or suspended due to poor or slow accrual. An unoptimized trial protocol, chronic and acute comorbidities (e.g., hypertension or diabetes) of participants, a low recruitment momentum, a slow screening process for the first trial participants, and a better alternative therapy may result in poor or slow accrual.459
(iii) Insufficient supply of nanomedicines. For instance, CCX872-B, a CCR2 inhibitor, was in shortage in a phase I/II trial (NCT03778879). Challenging issues with chemistry, manufacturing and control (CMC) of nanomedicines, a high manufacture cost, or an insufficient manufacturing capacity could contribute to inadequate supply of nanomedicines for clinical trials. Facile preparation of nanomedicines with simple chemistry or structures could be readily transferred to large-scale production. Great reproducibility, a high yield in a large scale, a high purity of pharmaceutic active ingredients, and fewer preparation steps are essential for the CMC of these nanomedicines in good manufacturing practice (GMP). Importantly, CQAs of nanomedicines in terms of potency and safety should be thoroughly studied.
(iv) Ineffective treatment and high toxicity. A low biological efficacy and CD19 CAR CTL persistence result in terminating a phase I/II trial (NCT01195480). A tumor lysate vaccine, Imiquimod, and RT were used in another phase I trial (NCT01400672) to treat diffuse intrinsic pontine glioma and glioblastoma multiforme and this trial terminated due to ineffective treatment and high toxicity.
Overall, many factors contribute to the successful clinical translation of a nanomedicine to a specific indication. Treatment effectiveness and tolerant toxicity are two critical factors. Currently, there are few clinical trials in nanomedicine-mediated radio-immunotherapy. An approach to combining clinically used radiation therapeutic methods and approved immunotherapeutic nanoformulation agents should be considered for nanomedicine-mediated radio-immunotherapy first, and this approach can accelerate the clinical translation of this new treatment and the ensuing other nanomedicine-assisted radio-immunotherapy. In addition, feasible and precise criteria, along with reliable nanomedicine-aided imaging methods to differentiate the disparity in the treatment response and predict the degree of toxic, adverse effects have not been established due to the slow response of immunotherapy and radiotherapy.
5.3 Challenges for clinical translation
Despite the inspiring results from preclinical studies and a few clinical trials, there are many challenges for clinical translation of the nanomedicines in radio-immunotherapy.Generally, good manufacturing practice (GMP), gram or kilogram-scale production, and facile, reproducible preparation procedures are the three major hurdles on the way of nanomedicines’ “from bench to clinic”.460,461 In the context of nanomedicine-mediated cancer radio-immunotherapy, there are other specific issues.
(i) Challenges in building in vitro cell assays. Since the combinational biological basis of radiotherapy and immunotherapy is in a systemic manner, conventional in vitro cell assays fail to evaluate their therapeutic efficacy. Establishment of specifically designed cellular assays is always time- and resource-consuming. For instance, the main procedure of the in vitro cross-presentation assay usually takes 1–2 weeks to complete, and this does not include the duration for isolating and stimulating bone marrow-derived dendritic cells and CD8+ T cells from the murine bone marrow or spleen.462
(ii) Lack of investigation of large-scale human tumor-derived samples in animal models. Due to the inherent limitation, murine cell lines are often employed for most pre-clinical studies in radio-immunotherapy. A few humanized murine models still experience issues, such as rejection, poor or low MHC-restricting antigen-specific immune response, and a complex modeling process, thus hampering their wide application.463,464 So far, there is a lack of large-scale studies on human tumor-derived cell lines or tissues or organoids in animal models. Effective humanized murine models could help in shortening the gap between preclinical studies and clinical trials or practices.
(iii) Simple design and facile preparation of nanomedicines. Several adjuvant agents or pharmaceutical preparations for cancer vaccines and inorganic radiosensitizers have undergone clinical trials.175,465,466 Their chemical structure is simple and their prepare process is readily implemented in a GMP environment, thus their pharmacokinetics, therapeutic efficacy, safety, and tolerability can be monitored or evaluated via currently established methods.
To conclude, this research area is still in its fetal period and its great potential remains to be explored. Great efforts need to be devoted to this research area. Currently, over-engineered nanomedicines in a controlled manner could provide a potential means of accelerating their clinical translation for simultaneously exploring the biological function and synergistic biological effect of multiple agents. After the mechanistic action of these over-engineered nanomedicines is revealed, their structure and preparation could be simplified. More importantly, personalized treatment in this emerging research field, consisting of nanotechnology-aided modification and optimized CAR T cells, DCs, tumor cells, or antigenic tumoral peptides obtained from patients’ biological samples, involves superfluous less controllable steps and has reproducible issues, but increasing evidence from these preclinical studies or clinical trials contribute to developing potent, simple, personalized nanomedicines.467,468
5.4 Future directions
Based on the challenging issues, lessons drawn from ongoing, completed, or withdrawn trials, along with recent advances in this field, we suggest three promising research topics on nanomedicine-assisted cancer radio-immunotherapy: radiolabelled immunogenic antibodies for theranostics, harnessing inflammation, and metastases-directed combined therapy.Additionally, expanding the repertoire of theranostic radionuclides can be critical for this treatment method. Visible isotopes of currently used therapeutic radionuclides may be such a candidate. For instance, thyroid tumor ablation was realized with the help of 131I, and liver cancer with 90Y microspheres. Their corresponding isotopes, such as 124I and 86Y, could be utilized to achieve imaging function.471 Other typical theranostic pairs include 64Cu/67Cu, 68Ga/67Ga, 83Sr/89Sr, 203Pb/212Pb, and 72As/77As.472 Their radioactive potencies and biological half-lives should be considered during the selection of these theranostic pairs: the half-lives should be slightly long enough to cover the duration including customized labelling, average cargo delivery, and peak tumor accumulation; and after adequate tumoral accumulation they can exert their tumor-killing effect in a rational time range without noticeable adverse effects. Chelator binding prevails in the current radiolabeling methods.473 In addition to clinically used chelators, such as 1,4,7,10-tetraazacyclododecane-tetraacetic acid (DOTA) and 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA), other tightly-bound and selective chelating ligands with high specific activities could be explored. Moreover, tumor-specific targeting moieties of these ligands should be modified to avoid potential damage to immune cells.
The inflammation process originating from RT may have “double-sword” impacts on cancer immunity. On the one hand, RT induces rapid inflammatory response via recruiting TAMs, leading to pseudo-progression and hindrance of timely and effective treatment.476 Furthermore, immune analysis reveals that incomplete radiofrequency ablation induces sustained local inflammation with predominant myeloid suppressor cells, which inhibit T cell function in tumors.477 On the other hand, RT-induced inflammation contributes to shaping the antigenicity of tumor cells, as well as the phenotype, immune-polarity, and density of inflammatory immune cells.476
Notably, inflammation can be a therapeutic target.478,479 Therapeutic intervention of chronic inflammation in tumors could enhance the clinical efficacy of therapeutic vaccines and adoptive T cells,480 because inflammation in the tumor can lead to caspase-1-dependent pyroptosis, a highly inflammatory form of programmed cell death.481,482 On basis of this finding, a few nanomedicines have been designed to augment inflammation. For example, the phospholipid-coated Na2S2O8 NP, an H2O2-independent ROS-generating agent, was in situ degraded to toxic ˙SO4− and ˙OH. The osmotic pressure induced by excess released Na+ from this peroxydisulfate NP led to caspase-1 dependent pyroptosis, evidenced by a significant increase in the caspase-1 activity and secretion of IL-1β in 4T1 and CT26 cells treated with this NP.483
Overall, inflammation could be harnessed during radio-immunotherapy of cancer. The inflammatory TME could be remodeled via RT, and anti-inflammation drugs are then used to avoid adverse events. However, the antitumor inflammatory effect is in a transient therapeutic window and anti-inflammation drugs should be administered to avoid long-term inflammation and inflammation-induced toxicity. A highly efficient indicator for the therapeutic window is to be established for maximizing the positive effect of therapy-inducing inflammation but minimizing its adverse effects.
Nanomedicine-mediated radio-immunotherapy could be potent to this deadly indication, and it could detain growth, regression, or recurrence of both primary and distant tumors.487,488 In a recent report, phosphatidylserine-coated liposomes loaded with cyclic guanosine monophosphate-adenosine monophosphate, NP-cGAMP, were aerosolized for inhalation administration to treat multifocal lung metastases in combination with fractionated RT (24 Gy/3 fractions). Physiochemically, this NP-cGAMP had a size of around 119 nm and a zeta potential of −41 mV. Two distinctive in vivo lung metastasis models were built through intravenously injected B16-OVA cells and orthotopically injected 4T1-Luc cells into mammary fat pads. Lung metastasis sites were significantly reduced in both tumor models after the combined treatment of NP-cGAMP and RT compared to other therapies. Monitoring of 4T1 lung metastases with 39 day IVIS and MRI revealed that the metastasis sites after this treatment were under well control and the ratio of CD8+ T/Treg in 4T1 lung metastases was improved.489
Currently, a CREKA (Cys–Arg–Glu–Lys–Ala) peptide, targeting fibrin–fibronectin complexes in the tumor stroma and vessel walls, has been widely used as a metastasis-specific ligand.490 After more therapeutic targets for metastases, including melanocortin 1 receptor for metastatic melanoma and leucine-rich alpha-2-glycoprotein 1,491,492 have been discovered and confirmed, other reliable and feasible targeting ligands can be thereafter designed and applied to modify nanomedicines for treating multiple metastases, especially the tiny one. In addition, tumor metabolism can be exploited as a potential target to halt tumor cell growth and prevent tumor seeding since a few metabolic pathways experience dynamic changes during tumor migration.493
6. Conclusions
A body of evidence from preclinical studies and clinical trials has indicated that cancer radio-immunotherapy has distinctive advantages over monotherapy in improving therapeutic outcomes and mitigating immune- or radio-induced toxicity. Furthermore, unique properties of nanomedicines, such as tumor/lymphatic system-specific targeting, radio-sensitization, accommodation of multiple immunomodulators, and size/shape/surface tunability, endow them with multiple indispensable and supplementary roles in this synergistic radio-immunotherapy. Among these roles, nanomedicine-mediated imaging could help in the differentiation of the tumor immune state, patient stratification, diagnosis, monitoring and timely assessment of therapeutic response and prediction of long-term clinical benefits. In addition, amplifying the tumoricidal effect and reducing therapy-induced toxicities to a tolerant level have been achieved through rational design of nanomedicines with flexible modification in overcoming therapy resistance, eliciting solid innate or adaptive immune response, reinvigorating cytotoxic effectors, and increasing immune-metabolic interplays.Although the working principles of synergistic radio-immunotherapy and mechanisms of reversing immune- and/or radio-suppression have been unveiled to some extent for the nanomedicines in animal models, there are many challenges in the transitional process of “from bench to clinic” for nanomedicines in radio-immunotherapy. A consortium convened from multi-disciplinary teams could be formed to establish the criteria for therapeutic response, clinical benefits, acceptable toxicity level, patient selection and the library of suitable nanomedicine candidates for cancer radio-immunotherapy.
Author contributions
H.-N. Li, Q. Luo, and K. Luo conceived the outline of this review. H.-N. Li and Q. Luo collected papers for writing. H.-N. Li drafted the manuscript and performed the creation of figures. H. Zhang, X.-L. Ma, and Z.-W. Gu helped revise the manuscript and discuss the content. Q.-Y. Gong and K. Luo supervised this project and finalized this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Fig. 1–5, 7b(i), 8, 10 and 13 and table of contents entry were created with BioRender.com. This work was financially supported by the National Natural Science Foundation of China (52073193, 51873120, 81621003), 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21013).Notes and references
- U. Wiedermann, E. Garner-Spitzer, Y. Chao, M. Maglakelidze, I. Bulat, A. Dechaphunkul, W. Arpornwirat, C. Charoentum, C. J. Yen, T. C. Yau, S. Tanasanvimon, J. Maneechavakajorn, A. Sookprasert, L. Y. Bai, W. C. Chou, T. Ungtrakul, M. Drinic, J. Tobias, C. C. Zielinski, L. Chong, N. J. Ede, M. T. Marino and A. J. Good, Clin. Cancer Res., 2021, 27, 3649–3660 CrossRef CAS PubMed.
- G. Terrero, J. Datta, J. Dennison, D. A. Sussman, I. Lohse, N. B. Merchant and P. J. Hosein, JAMA Oncol., 2022, 8, 938–940 CrossRef.
- J. Gao, N. Navai, O. Alhalabi, A. Siefker-Radtke, M. T. Campbell, R. S. Tidwell, C. C. Guo, A. M. Kamat, S. F. Matin, J. C. Araujo, A. Y. Shah, P. Msaouel, P. Corn, J. Wang, J. N. Papadopoulos, S. S. Yadav, J. M. Blando, F. Duan, S. Basu, W. Liu, Y. Shen, Y. Zhang, M. D. Macaluso, Y. Wang, J. Chen, J. Zhang, A. Futreal, C. Dinney, J. P. Allison, S. Goswami and P. Sharma, Nat. Med., 2020, 26, 1845–1851 CrossRef CAS PubMed.
- N. K. Altorki, T. E. McGraw, A. C. Borczuk, A. Saxena, J. L. Port, B. M. Stiles, B. E. Lee, N. J. Sanfilippo, R. J. Scheff, B. B. Pua, J. F. Gruden, P. J. Christos, C. Spinelli, J. Gakuria, M. Uppal, B. Binder, O. Elemento, K. V. Ballman and S. C. Formenti, Lancet Oncol., 2021, 22, 824–835 CrossRef CAS.
- M. Hamieh, A. Dobrin, A. Cabriolu, S. J. C. van der Stegen, T. Giavridis, J. Mansilla-Soto, J. Eyquem, Z. Zhao, B. M. Whitlock, M. M. Miele, Z. Li, K. M. Cunanan, M. Huse, R. C. Hendrickson, X. Wang, I. Rivière and M. Sadelain, Nature, 2019, 568, 112–116 CrossRef CAS PubMed.
- B. Blanco, C. Domínguez-Alonso and L. Alvarez-Vallina, Clin. Cancer Res., 2021, 27, 5457–5464 CrossRef CAS PubMed.
- Y. Otani, J. Y. Yoo, C. T. Lewis, S. Chao, J. Swanner, T. Shimizu, J. M. Kang, S. A. Murphy, K. Rivera-Caraballo, B. Hong, J. C. Glorioso, H. Nakashima, S. E. Lawler, Y. Banasavadi-Siddegowda, J. D. Heiss, Y. Yan, G. Pei, M. A. Caligiuri, Z. Zhao, E. A. Chiocca, J. Yu and B. Kaur, Clin. Cancer Res., 2022, 28, 1460–1473 CrossRef CAS.
- W. Li, X. Zhang, C. Zhang, J. Yan, X. Hou, S. Du, C. Zeng, W. Zhao, B. Deng, D. W. McComb, Y. Zhang, D. D. Kang, J. Li, W. E. Carson III and Y. Dong, Nat. Commun., 2021, 12, 7264 CrossRef CAS.
- D. Xue, B. Moon, J. Liao, J. Guo, Z. Zou, Y. Han, S. Cao, Y. Wang, Y. X. Fu and H. Peng, Sci. Immunol., 2022, 7, eabi6899 CrossRef CAS.
- B. J. Schneider, J. Naidoo, B. D. Santomasso, C. Lacchetti, S. Adkins, M. Anadkat, M. B. Atkins, K. J. Brassil, J. M. Caterino, I. Chau, M. J. Davies, M. S. Ernstoff, L. Fecher, M. Ghosh, I. Jaiyesimi, J. S. Mammen, A. Naing, L. J. Nastoupil, T. Phillips, L. D. Porter, C. A. Reichner, C. Seigel, J. M. Song, A. Spira, M. Suarez-Almazor, U. Swami, J. A. Thompson, P. Vikas, Y. Wang, J. S. Weber, P. Funchain and K. Bollin, J. Clin. Oncol., 2021, 39, 4073–4126 CrossRef CAS PubMed.
- C. Valero, M. Lee, D. Hoen, A. Zehir, M. F. Berger, V. E. Seshan, T. A. Chan and L. G. T. Morris, JAMA Oncol., 2021, 7, 739–743 CrossRef PubMed.
- K. B. Pointer, S. P. Pitroda and R. R. Weichselbaum, Trends Cancer, 2022, 8, 9–20 CrossRef CAS.
- J. D. Schoenfeld, A. Giobbie-Hurder, S. Ranasinghe, K. Z. Kao, A. Lako, J. Tsuji, Y. Liu, R. C. Brennick, R. D. Gentzler, C. Lee, J. Hubbard, S. M. Arnold, J. L. Abbruzzese, S. K. Jabbour, N. V. Uboha, K. L. Stephans, J. M. Johnson, H. Park, L. C. Villaruz, E. Sharon, H. Streicher, M. M. Ahmed, H. Lyon, C. Cibuskis, N. Lennon, A. Jhaveri, L. Yang, J. Altreuter, L. Gunasti, J. L. Weirather, R. H. Mak, M. M. Awad, S. J. Rodig, H. X. Chen, C. J. Wu, A. M. Monjazeb and F. S. Hodi, Lancet Oncol., 2022, 23, 279–291 CrossRef CAS.
- S. George, B. I. Rini and H. J. Hammers, JAMA Oncol., 2019, 5, 411–421 CrossRef PubMed.
- A. Gomez-Iturriaga, M. Keyes, J. Martin and D. E. Spratt, Lancet Oncol., 2022, 23, 23–25 CrossRef.
- N. G. Zaorsky, J. B. Yu, S. M. McBride, R. T. Dess, W. C. Jackson, B. A. Mahal, R. Chen, A. Choudhury, A. Henry, I. Syndikus, T. Mitin, A. Tree, A. U. Kishan and D. E. Spratt, Adv. Radiat. Oncol., 2020, 5, 26–32 CrossRef.
- M. E. Rodríguez-Ruiz, C. Vanpouille-Box, I. Melero, S. C. Formenti and S. Demaria, Trends Immunol., 2018, 39, 644–655 CrossRef.
- S. Rottenberg, C. Disler and P. Perego, Nat. Rev. Cancer, 2021, 21, 37–50 CrossRef CAS.
- H. B. Barsoumian, R. Ramapriyan, A. I. Younes, M. S. Caetano, H. Menon, N. I. Comeaux, T. R. Cushman, J. E. Schoenhals, A. P. Cadena, T. P. Reilly, D. Chen, F. Masrorpour, A. Li, D. S. Hong, A. Diab, Q. N. Nguyen, I. Glitza, R. Ferrarotto, S. G. Chun, M. A. Cortez and J. Welsh, J. Immunother. Cancer, 2020, 8, e000537 CrossRef.
- S. S. Du, G. W. Chen, P. Yang, Y. X. Chen, Y. Hu, Q. Q. Zhao, Y. Zhang, R. Liu, D. X. Zheng, J. Zhou, J. Fan and Z. C. Zeng, Int. J. Radiat. Oncol., Biol., Phys., 2022, 112, 1243–1255 CrossRef.
- F. G. Herrera, C. Ronet, M. Ochoa de Olza, D. Barras, I. Crespo, M. Andreatta, J. Corria-Osorio, A. Spill, F. Benedetti, R. Genolet, A. Orcurto, M. Imbimbo, E. Ghisoni, B. Navarro Rodrigo, D. R. Berthold, A. Sarivalasis, K. Zaman, R. Duran, C. Dromain, J. Prior, N. Schaefer, J. Bourhis, G. Dimopoulou, Z. Tsourti, M. Messemaker, T. Smith, S. E. Warren, P. Foukas, S. Rusakiewicz, M. J. Pittet, S. Zimmermann, C. Sempoux, U. Dafni, A. Harari, L. E. Kandalaft, S. J. Carmona, D. Dangaj Laniti, M. Irving and G. Coukos, Cancer Discovery, 2022, 12, 108–133 CrossRef CAS.
- R. J. Griffin, M. M. Ahmed, B. Amendola, O. Belyakov, S. M. Bentzen, K. T. Butterworth, S. Chang, C. N. Coleman, V. Djonov, S. C. Formenti, E. Glatstein, C. Guha, S. Kalnicki, Q. T. Le, B. W. Loo, Jr., A. Mahadevan, M. Massaccesi, P. G. Maxim, M. Mohiuddin, M. Mohiuddin, N. A. Mayr, C. Obcemea, K. Petersson, W. Regine, M. Roach, P. Romanelli, C. B. Simone 2nd, J. W. Snider, D. R. Spitz, B. Vikram, M. C. Vozenin, M. Abdel-Wahab, J. Welsh, X. Wu and C. L. Limoli, Int. J. Radiat. Oncol. Biol. Phys., 2020, 107, 766–778 CrossRef.
- S. Demaria, C. Guha, J. Schoenfeld, Z. Morris, A. Monjazeb, A. Sikora, M. Crittenden, S. Shiao, S. Khleif, S. Gupta, S. C. Formenti, B. Vikram, C. N. Coleman and M. M. Ahmed, J. Immunother. Cancer, 2021, 9, e002038 CrossRef.
- S. P. Keam, H. Halse, T. Nguyen, M. Wang, N. Van Kooten Losio, C. Mitchell, F. Caramia, D. J. Byrne, S. Haupt, G. Ryland, P. K. Darcy, S. Sandhu, P. Blombery, Y. Haupt, S. G. Williams and P. J. Neeson, J. Immunother. Cancer, 2020, 8, e000792 CrossRef.
- M. A. Postow, M. K. Callahan, C. A. Barker, Y. Yamada, J. Yuan, S. Kitano, Z. Mu, T. Rasalan, M. Adamow and E. Ritter, New Engl. J. Med., 2012, 366, 925–931 CrossRef CAS PubMed.
- W. Ngwa, O. C. Irabor, J. D. Schoenfeld, J. Hesser, S. Demaria and S. C. Formenti, Nat. Rev. Cancer, 2018, 18, 313–322 CrossRef CAS.
- J. S. O'Donnell, M. W. L. Teng and M. J. Smyth, Nat. Rev. Clin. Oncol., 2019, 16, 151–167 CrossRef.
- A. X. Lozano, A. A. Chaudhuri, A. Nene, A. Bacchiocchi, N. Earland, M. D. Vesely, A. Usmani, B. E. Turner, C. B. Steen, B. A. Luca, T. Badri, G. S. Gulati, M. R. Vahid, F. Khameneh, P. K. Harris, D. Y. Chen, K. Dhodapkar, M. Sznol, R. Halaban and A. M. Newman, Nat. Med., 2022, 28, 353–362 CrossRef CAS.
- N. Gabriel, K. Balaji, K. Jayachandran, M. Inkman, J. Zhang, S. Dahiya and M. Goldstein, Cancer Res., 2022, 82, 2019–2030 CrossRef CAS.
- S. B. Elsherif, M. Anderson, A. A. Chaudhry, S. P. Kumar, D. R. Gopireddy, C. Lall and P. R. Bhosale, Eur. J. Radiol., 2022, 146, 110062 CrossRef.
- Q. Luo, Z. Duan, X. Li, L. Gu, L. Ren, H. Zhu, X. Tian, R. Chen, H. Zhang, Q. Gong, Z. Gu and K. Luo, Adv. Funct. Mater., 2022, 32, 2110408 CrossRef CAS.
- X. Zheng, D. Pan, G. Zhu, L. Zhang, A. Bhamra, R. Chen, H. Zhang, Q. Gong, Z. Gu and K. Luo, Adv. Mater., 2022, 34, 2201200 CrossRef CAS PubMed.
- M. B. Atkins, M. J. Robertson, M. Gordon, M. T. Lotze, M. DeCoste, J. S. DuBois, J. Ritz, A. B. Sandler, H. D. Edington, P. D. Garzone, J. W. Mier, C. M. Canning, L. Battiato, H. Tahara and M. L. Sherman, Clin. Cancer Res., 1997, 3, 409–417 CAS.
- H. Cai, Y. Xiang, Y. Zeng, Z. Li, X. Zheng, Q. Luo, H. Zhu, Q. Gong, Z. Gu, Y. Liu, H. Zhang and K. Luo, Acta Pharm. Sin. B, 2021, 11, 544–559 CrossRef CAS PubMed.
- S. S. Sridhar, N. Blais, B. Tran, M. N. Reaume, S. A. North, M. R. Stockler, K. N. Chi, N. E. Fleshner, G. Liu, J. W. Robinson, S. D. Mukherjee, Y. Rahim, E. Winquist, C. M. Booth, N. T. Nguyen, E. K. Beardsley, N. S. Alimohamed, G. T. McDonald, K. Ding and W. R. Parulekar, JAMA Oncol., 2020, 6, 1751–1758 CrossRef PubMed.
- H. Park, A. Otte and K. Park, J. Controlled Release, 2022, 342, 53–65 CrossRef CAS.
- E. Pujade-Lauraine, K. Fujiwara, J. A. Ledermann, A. M. Oza, R. Kristeleit, I.-L. Ray-Coquard, G. E. Richardson, C. Sessa, K. Yonemori and S. Banerjee, Lancet Oncol., 2021, 22, 1034–1046 CrossRef CAS.
- C. Ji, M. Zhao, C. Wang, R. Liu, S. Zhu, X. Dong, C. Su and Z. Gu, ACS Nano, 2022, 16, 9428–9441 CrossRef CAS.
- H. Song, H. Sun, N. He, C. Xu, Y. Wang, L. Du, Y. Liu, Q. Wang, K. Ji, J. Wang, M. Zhang, Y. Gu, Y. Zhang, L. Feng, O. Tillement, W. Wang and Q. Liu, Nanoscale, 2022, 14, 11429–11442 RSC.
- T. L. Nguyen, B. G. Cha, Y. Choi, J. Im and J. Kim, Biomaterials, 2020, 239, 119859 CrossRef CAS.
- C. A. Ferreira, S. Goel, E. B. Ehlerding, Z. T. Rosenkrans, D. Jiang, T. Sun, E. Aluicio-Sarduy, J. W. Engle, D. Ni and W. Cai, Nano Lett., 2021, 21, 4692–4699 CrossRef CAS PubMed.
- Q. Hu, H. Li, E. Archibong, Q. Chen, H. Ruan, S. Ahn, E. Dukhovlinova, Y. Kang, D. Wen, G. Dotti and Z. Gu, Nat. Biomed. Eng., 2021, 5, 1038–1047 CrossRef CAS.
- F. G. Herrera, J. Bourhis and G. Coukos, Ca-Cancer J. Clin., 2017, 67, 65–85 CrossRef.
- J. C. Jagodinsky, P. M. Harari and Z. S. Morris, Int. J. Radiat. Oncol. Biol. Phys., 2020, 108, 6–16 CrossRef.
- H. Zhuang, Cancer Commun., 2020, 40, 649–654 CrossRef.
- M. Trommer, S. Y. Yeo, T. Persigehl, A. Bunck, H. Grüll, M. Schlaak, S. Theurich, M. von Bergwelt-Baildon, J. Morgenthaler and J. M. Herter, Front. Pharmacol., 2019, 10, 511 CrossRef CAS PubMed.
- S. Demaria, B. Ng, M. L. Devitt, J. S. Babb, N. Kawashima, L. Liebes and S. C. Formenti, Int. J. Radiat. Oncol. Biol. Phys., 2004, 58, 862–870 CrossRef PubMed.
- T. P. Lippert and R. A. Greenberg, J. Clin. Invest., 2021, 131, e148274 CrossRef CAS PubMed.
- L. Zhu, S. Hu, Q. Chen, H. Zhang, J. Fu, Y. Zhou, Y. Bai, Y. Pan and C. Shao, Int. J. Biol. Sci., 2021, 17, 926–941 CrossRef CAS.
- W. Wang, H. Xu, Q. Ye, F. Tao, I. Wheeldon, A. Yuan, Y. Hu and J. Wu, Nat. Biomed. Eng., 2022, 6, 44–53 CrossRef CAS.
- E. Mizukoshi, H. Nakagawa, T. Tamai, M. Kitahara, K. Fushimi, K. Nio, T. Terashima, N. Iida, K. Arai, T. Yamashita, T. Yamashita, Y. Sakai, M. Honda and S. Kaneko, Nat. Commun., 2022, 13, 3123 CrossRef CAS.
- C. Lhuillier, N. P. Rudqvist, T. Yamazaki, T. Zhang, M. Charpentier, L. Galluzzi, N. Dephoure, C. C. Clement, L. Santambrogio, X. K. Zhou, S. C. Formenti and S. Demaria, J. Clin. Invest., 2021, 131, e138740 CrossRef CAS PubMed.
- S. C. Formenti, N.-P. Rudqvist, E. Golden, B. Cooper, E. Wennerberg, C. Lhuillier, C. Vanpouille-Box, K. Friedman, L. F. de Andrade and K. W. Wucherpfennig, Nat. Med., 2018, 24, 1845–1851 CrossRef CAS PubMed.
- Z. Tuo, Q. He, Z. Zhang, Y. Wang, J. Sun, Q. Wei, Y. Hu, J. F. Lovell, H. Jin and K. Yang, Chem. Eng. J., 2022, 433, 134437 CrossRef CAS.
- Z. Bian, L. Shi, K. Kidder, K. Zen, C. Garnett-Benson and Y. Liu, Nat. Commun., 2021, 12, 3229 CrossRef CAS.
- K. M. Garland, T. L. Sheehy and J. T. Wilson, Chem. Rev., 2022, 122, 5977–6039 CrossRef CAS.
- H. du Bois, T. A. Heim and A. W. Lund, Sci. Immunol., 2021, 6, eabg3551 CrossRef CAS PubMed.
- H. Liang, L. Deng, Y. Hou, X. Meng, X. Huang, E. Rao, W. Zheng, H. Mauceri, M. Mack, M. Xu, Y. Fu and R. R. Weichselbaum, Nat. Commun., 2017, 8, 1736 CrossRef PubMed.
- N.-P. Rudqvist, K. A. Pilones, C. Lhuillier, E. Wennerberg, J.-W. Sidhom, R. O. Emerson, H. S. Robins, J. Schneck, S. C. Formenti and S. Demaria, Cancer Immunol. Res., 2018, 6, 139–150 CrossRef CAS PubMed.
- M. O. de Olza, B. N. Rodrigo, S. Zimmermann and G. Coukos, Lancet Oncol., 2020, 21, 419–430 CrossRef.
- A. Arina, S. I. Gutiontov and R. R. Weichselbaum, Clin. Cancer Res., 2020, 26, 2777–2782 CrossRef CAS.
- J. N. Cheng, W. Luo, C. Sun, Z. Jin, X. Zeng, P. B. Alexander, Z. Gong, X. Xia, X. Ding, S. Xu, P. Zou, Y. Y. Wan, Q. Jia, Q. J. Li and B. Zhu, Sci. Adv., 2021, 7, eabc7609 CrossRef CAS.
- W. Cao, G. Chen, L. Wu, K. N. Yu, M. Sun, M. Yang, Y. Jiang, Y. Jiang, Y. Xu, S. Peng and W. Han, Int. J. Radiat. Oncol. Biol. Phys., 2022 DOI:10.1016/j.ijrobp.2022.07.1841.
- A. Arina, M. Beckett, C. Fernandez, W. Zheng, S. Pitroda, S. J. Chmura, J. J. Luke, M. Forde, Y. Hou and B. Burnette, Nat. Commun., 2019, 10, 3959 CrossRef PubMed.
- S. Murty, S. T. Haile, C. Beinat, A. Aalipour, I. S. Alam, T. Murty, T. M. Shaffer, C. B. Patel, E. E. Graves and C. L. Mackall, OncoImmunology, 2020, 9, 1757360 CrossRef.
- E. D. Brooks and J. Y. Chang, Nat. Rev. Clin. Oncol., 2019, 16, 123–135 CrossRef.
- A. J. Oweida, L. Darragh, A. Phan, D. Binder, S. Bhatia, A. Mueller, B. V. Court, D. Milner, D. Raben and R. Woessner, J. Natl. Cancer Inst., 2019, 111, 1339–1349 CrossRef CAS PubMed.
- Y. Hou, H. L. Liang, X. Yu, Z. Liu, X. Cao, E. Rao, X. Huang, L. Wang, L. Li and J. Bugno, Sci. Transl. Med., 2021, 13, eabb0130 CrossRef CAS.
- D. F. Boreel, P. N. Span, S. Heskamp, G. J. Adema and J. Bussink, Clin. Cancer Res., 2021, 27, 2970–2978 CrossRef CAS.
- I. Minn, S. P. Rowe and M. G. Pomper, Lancet Oncol., 2019, 20, 443–451 CrossRef PubMed.
- R. S. Goedegebuure, C. Vonk, L. P. Kooij, S. Derks and V. L. Thijssen, Int. J. Radiat. Oncol. Biol. Phys., 2020, 108, 56–69 CrossRef.
- C. Xie, A. G. Duffy, G. Brar, S. Fioravanti, D. Mabry-Hrones, M. Walker, C. M. Bonilla, B. J. Wood, D. E. Citrin, E. M. Gil Ramirez, F. E. Escorcia, B. Redd, J. M. Hernandez, J. L. Davis, B. Gasmi, D. Kleiner, S. M. Steinberg, J. C. Jones and T. F. Greten, Clin. Cancer Res., 2020, 26, 2318–2326 CrossRef CAS PubMed.
- W. Theelen, H. M. U. Peulen, F. Lalezari, V. van der Noort, J. F. de Vries, J. Aerts, D. W. Dumoulin, I. Bahce, A. N. Niemeijer, A. J. de Langen, K. Monkhorst and P. Baas, JAMA Oncol., 2019, 5, 1276–1282 CrossRef PubMed.
- S. C. Katz, E. Prince, M. Cunetta, P. Guha, A. Moody, V. Armenio, L. J. Wang, N. J. Espat and R. P. Junghans, Cancer Res., 2017, 77, CT109 CrossRef.
- S. Modak, P. Zanzonico, M. Grkovski, E. K. Slotkin, J. A. Carrasquillo, S. K. Lyashchenko, J. S. Lewis, I. Y. Cheung, T. Heaton, M. P. LaQuaglia, N. V. Cheung and N. Pandit-Taskar, J. Clin. Oncol., 2020, 38, 4283–4291 Search PubMed.
- C. Bodet-Milin, L. Ferrer, A. Rauscher, D. Masson, L. Rbah-Vidal, A. Faivre-Chauvet, E. Cerato, C. Rousseau, J. Hureaux, O. Couturier, P. Y. Salaün, D. M. Goldenberg, R. M. Sharkey, F. Kraeber-Bodéré and J. Barbet, Front. Med.-Lausanne, 2015, 2, 84 Search PubMed.
- P. Twardowski, J. Y. C. Wong, S. K. Pal, P. H. Frankel, K. Franklin, M. Junqueira, M. R. Prajapati, D. Harwood and N. Agarwal, J. Clin. Oncol., 2017, 35, 222 Search PubMed.
- C. H. Marshall, J. C. Park, T. L. DeWeese, S. King, M. Afful, J. Hurrelbrink, C. Manogue, P. Cotogno, N. P. Moldawer, P. C. Barata, C. G. Drake, E. M. Posadas, A. J. Armstrong, A. O. Sartor and E. S. Antonarakis, J. Clin. Oncol., 2020, 38, 130 Search PubMed.
- C. R. Heery, R. A. Madan, M. Bilusic, J. W. Kim, N. K. Singh, M. Rauckhorst, S. M. Steinberg, W. L. Dahut, C. Chen, R. S. DiPaola, M. N. Stein, D. Panicali, J. W. Hodge, J. Schlom and J. L. Gulley, J. Clin. Oncol., 2013, 31, 102 Search PubMed.
- Y. D. Seo, J. Zhou, K. Morse, J. Patino, S. Mackay, E. Y. Kim, E. U. Conrad, R. B. O'Malley, L. D. Cranmer, H. Lu, F. J. Hsu, Y. Xu, E. T. Loggers, T. Hain, V. G. Pillarisetty, G. Kane, S. Riddell, J. H. ter Meulen, R. L. Jones and S. Pollack, J. Clin. Oncol., 2018, 36, 71 Search PubMed.
- M. D. Janosky, S. Demaria, Y. Novik, R. Oratz, A. Tiersten, J. D. Goldberg, E. Wang, F. Marincola, S. Formenti and S. Adams, J. Clin. Oncol., 2013, 31, TPS3122 Search PubMed.
- E. J. Van Limbergen, A. Hoeben, R. I. Y. Lieverse, R. Houben, C. Overhof, A. Postma, J. Zindler, F. Verhelst, L. J. Dubois, D. De Ruysscher, E. G. C. Troost and P. Lambin, Int. J. Radiat. Oncol. Biol. Phys., 2021, 109, 1421–1430 CrossRef.
- R. I. Y. Lieverse, E. J. Van Limbergen, C. J. G. Oberije, E. G. C. Troost, S. R. Hadrup, A. C. Dingemans, L. E. L. Hendriks, F. Eckert, C. Hiley, C. Dooms, Y. Lievens, M. C. de Jong, J. Bussink, X. Geets, V. Valentini, G. Elia, D. Neri, C. Billiet, A. Abdollahi, D. Pasquier, P. Boisselier, A. Yaromina, D. De Ruysscher, L. J. Dubois and P. Lambin, BMC Cancer, 2020, 20, 557 CrossRef CAS PubMed.
- V. Olivo Pimentel, D. Marcus, A. M. van der Wiel, N. G. Lieuwes, R. Biemans, R. I. Lieverse, D. Neri, J. Theys, A. Yaromina, L. J. Dubois and P. Lambin, J. Immunother. Cancer, 2021, 9, e001764 CrossRef.
- P. Gabani, B. W. Fischer-Valuck, T. M. Johanns, L. F. Hernandez-Aya, J. W. Keller, K. M. Rich, A. H. Kim, G. P. Dunn, C. G. Robinson and M. R. Chicoine, Radiother. Oncol., 2018, 128, 266–273 Search PubMed.
- J. E. Bates, C. G. Morris, M. T. Milano, A. R. Yeung and B. S. Hoppe, Radiother. Oncol., 2019, 138, 75–79 CrossRef PubMed.
- J. Nam, S. Son, K. S. Park, W. Zou, L. D. Shea and J. J. Moon, Nat. Rev. Mater., 2019, 4, 398–414 Search PubMed.
- D. J. Irvine and E. L. Dane, Nat. Rev. Immunol., 2020, 20, 321–334 CrossRef CAS.
- J. Lu, Z. Guo, R. Zheng, W. Xie, X. Gao, J. Gao, Y. Zhang, W. Xu, J. Ye, X. Guo, J. Tang, J. Yu, L. Wang, B. Xu, G. Zhang and L. Zhao, ACS Appl. Mater. Interfaces, 2022, 14, 4995–5008 Search PubMed.
- Y. Li, K. Zhang, Y. Wu, Y. Yue, K. Cheng, Q. Feng, X. Ma, J. Liang, N. Ma, G. Liu, G. Nie, L. Ren and X. Zhao, Small, 2022, 18, 2107461 Search PubMed.
- C. Czysch, C. Medina-Montano, N. K. Dal, T. Dinh, Y. Fröder, P. Winterwerber, K. Maxeiner, H. J. Räder, D. Schuppan, H. Schild, M. Bros, B. Biersack, F. Feranoli, S. Grabbe and L. Nuhn, Macromol. Rapid Commun., 2022, 43, 2200095 CrossRef CAS.
- H. Liu, Z. Wen, H. Chen, Z. Yang, Z. Le, Z. Liu, Y. Chen and L. Liu, J. Controlled Release, 2022, 352, 497–506 CrossRef CAS.
- M. Wehbe, L. Wang-Bishop, K. W. Becker, D. Shae, J. J. Baljon, X. He, P. Christov, K. L. Boyd, J. M. Balko and J. T. Wilson, J. Controlled Release, 2021, 330, 1118–1129 Search PubMed.
- H. Zheng, B. Guo, X. Qiu, Y. Xia, Y. Qu, L. Cheng, F. Meng and Z. Zhong, Bioact. Mater., 2022, 16, 1–11 CrossRef CAS.
- C. Wang, Z. Dong, Y. Hao, Y. Zhu, J. Ni, Q. Li, B. Liu, Y. Han, Z. Yang, J. Wan, K. Yang, Z. Liu and L. Feng, Adv. Mater., 2022, 34, 2106520 CrossRef CAS.
- A. Mansurov, P. Hosseinchi, K. Chang, A. L. Lauterbach, L. T. Gray, A. T. Alpar, E. Budina, A. J. Slezak, S. Kang, S. Cao, A. Solanki, S. Gomes, J. M. Williford, M. A. Swartz, J. L. Mendoza, J. Ishihara and J. A. Hubbell, Nat. Biomed. Eng., 2022, 6, 819–829 CrossRef CAS.
- F. Zamboni, S. Vieira, R. L. Reis, J. Miguel Oliveira and M. N. Collins, Prog. Mater. Sci., 2018, 97, 97–122 Search PubMed.
- Y. Zeng, Y. Xiang, R. Sheng, H. Tomás, J. Rodrigues, Z. Gu, H. Zhang, Q. Gong and K. Luo, Bioact. Mater., 2021, 6, 3358–3382 Search PubMed.
- R. I. Jakacki, E. Dombi, S. M. Steinberg, S. Goldman, M. W. Kieran, N. J. Ullrich, I. F. Pollack, A. Goodwin, P. E. Manley, J. Fangusaro, R. Allen and B. C. Widemann, Neuro. Oncol., 2017, 19, 289–297 CAS.
- Y. Yu, J. Li, B. Song, Z. Ma, Y. Zhang, H. Sun, X. Wei, Y. Bai, X. Lu, P. Zhang and X. Zhang, Biomaterials, 2022, 280, 121312 CrossRef CAS.
- J. Bu, A. Nair, M. Iida, W. J. Jeong, M. J. Poellmann, K. Mudd, L. J. Kubiatowicz, E. W. Liu, D. L. Wheeler and S. Hong, Nano Lett., 2020, 20, 4901–4909 CrossRef CAS.
- W. J. Jeong, J. Bu, Y. Han, A. J. Drelich, A. Nair, P. Král and S. Hong, J. Am. Chem. Soc., 2020, 142, 1832–1837 CrossRef CAS.
- A. Mansurov, P. Hosseinchi, K. Chang, A. L. Lauterbach, L. T. Gray, A. T. Alpar, E. Budina, A. J. Slezak, S. Kang and S. Cao, Nat. Biomed. Eng., 2022, 6, 819–829 CrossRef CAS.
- I. Etxeberria, E. Bolaños, A. Teijeira, S. Garasa, A. Yanguas, A. Azpilikueta, W. M. Kavanaugh, O. Vasiljeva, M. Belvin, B. Howng, B. Irving, K. Tipton, J. West, L. Mei, A. J. Korman, E. Sega, I. Olivera, A. Cirella, M. C. Ochoa, M. E. Rodriguez, A. Melero, M. F. Sanmamed, J. J. Engelhardt and I. Melero, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2025930118 CrossRef CAS.
- L. Tang, Y. Zheng, M. B. Melo, L. Mabardi, A. P. Castaño, Y. Q. Xie, N. Li, S. B. Kudchodkar, H. C. Wong, E. K. Jeng, M. V. Maus and D. J. Irvine, Nat. Biotechnol., 2018, 36, 707–716 CrossRef CAS PubMed.
- C. W. Shields IV, M. A. Evans, L. L. Wang, N. Baugh, S. Iyer, D. Wu, Z. Zhao, A. Pusuluri, A. Ukidve, D. C. Pan and S. Mitragotri, Sci. Adv., 2020, 6, eaaz6579 CrossRef.
- D. Manoharan, L. C. Chang, L. C. Wang, Y. S. Shan, F. C. Lin, L. C. Wu, H. S. Sheu, W. P. Su and C. S. Yeh, ACS Nano, 2021, 15, 9084–9100 Search PubMed.
- P. Zhang, A. Darmon, J. Marill, N. M. Anesary and S. Paris, Int. J. Nanomed., 2020, 15, 3843–3850 CrossRef CAS.
- L. Zheng, R. Zhu, L. Chen, Q. Fu, J. Li, C. Chen, J. Song and H. Yang, Nano Res., 2021, 14, 3744–3755 CrossRef CAS.
- P. Pan, X. Dong, Y. Chen, X. Zeng and X. Z. Zhang, ACS Nano, 2022, 16, 801–812 CrossRef CAS.
- L. Maggiorella, G. Barouch, C. Devaux, A. Pottier, E. Deutsch, J. Bourhis, E. Borghi and L. Levy, Future Oncol., 2012, 8, 1167–1181 CrossRef CAS.
- Y. Chen, P. Gao, T. Wu, W. Pan, N. Li and B. Tang, Chem. Commun., 2020, 56, 10621–10630 RSC.
- J. Xue, D. Duosiken, S. Zhong, J. J. Cao, L. Y. Hu, K. Sun, K. Tao and S. J. Pan, Acta Biomater., 2021, 131, 508–518 Search PubMed.
- H. Deng, Z. Zhou, W. Yang, L. S. Lin, S. Wang, G. Niu, J. Song and X. Chen, Nano Lett., 2020, 20, 1928–1933 Search PubMed.
- Z. Duan, Q. Luo, L. Gu, X. Li, H. Zhu, Z. Gu, Q. Gong, H. Zhang and K. Luo, Nanoscale, 2021, 13, 13681–13692 Search PubMed.
- Z. Lu, S. Bai, Y. Jiang, S. Wu, D. Xu, Y. Chen, Y. Lan, Y. An, J. Mao, X. Liu and G. Liu, Adv. Funct. Mater., 2022, 32, 2207749 CrossRef CAS.
- C. Du, M. Zhou, F. Jia, L. Ruan, H. Lu, J. Zhang, B. Zhu, X. Liu, J. Chen, Z. Chai and Y. Hu, Biomaterials, 2021, 269, 120642 CrossRef CAS.
- M. Jiang, B. Qin, L. Luo, X. Li, Y. Shi, J. Zhang, Z. Luo, C. Zhu, G. Guan, Y. Du and J. You, J. Controlled Release, 2021, 335, 408–419 CrossRef CAS.
- X. Zhou, M. You, F. Wang, Z. Wang, X. Gao, C. Jing, J. Liu, M. Guo, J. Li, A. Luo, H. Liu, Z. Liu and C. Chen, Adv. Mater., 2021, 33, 2100556 Search PubMed.
- S. de Leve, F. Wirsdörfer and V. Jendrossek, Front. Immunol., 2019, 10, 698 CrossRef CAS.
- W. Li, J. Yan, H. Tian, B. Li, G. Wang, W. Sang, Z. Zhang, X. Zhang and Y. Dai, Bioact. Mater., 2023, 22, 34–46 CrossRef CAS.
- Y. Jiang, J. Martin, M. Alkadhimi, K. Shigemori, P. Kinchesh, S. Gilchrist, V. Kersemans, S. Smart, J. M. Thompson, M. A. Hill, M. J. O'Connor, B. R. Davies and A. J. Ryan, Br. J. Cancer, 2021, 124, 1809–1819 CrossRef CAS PubMed.
- S. R. Vink, S. Lagerwerf, E. Mesman, J. H. Schellens, A. C. Begg, W. J. van Blitterswijk and M. Verheij, Clin. Cancer Res., 2006, 12, 1615–1622 CrossRef CAS.
- M. Landry, D. Nelson, E. Choi, A. DuRoss and C. Sun, Transl. Oncol., 2022, 16, 101336 CrossRef CAS PubMed.
- S. Clement, J. M. Campbell, W. Deng, A. Guller, S. Nisar, G. Liu, B. C. Wilson and E. M. Goldys, Adv. Sci., 2020, 7, 2003584 Search PubMed.
- T. Luo, G. T. Nash, X. Jiang, X. Feng, J. Mao, J. Liu, A. Juloori, A. T. Pearson and W. Lin, Adv. Mater., 2022, 34, 2110588 CrossRef CAS PubMed.
- D. Wang, T. Nie, C. Huang, Z. Chen, X. Ma, W. Fang, Y. Huang, L. Luo and Z. Xiao, Small, 2022, 18, 2203227 CrossRef CAS.
- J. Shi, K. Xu, A. Keyvanloo, T. S. Udayakumar, A. Ahmad, F. Yang and Y. Yang, Int. J. Radiat. Oncol. Biol. Phys., 2020, 108, 1063–1072 CrossRef PubMed.
- K. R. Patel, A. Martinez, J. M. Stahl, S. J. Logan, A. J. Perricone, M. J. Ferris, Z. S. Buchwald, M. Chowdhary, K. A. Delman, D. K. Monson, S. V. Oskouei, N. B. Reimer, K. Cardona, M. A. Edgar and K. D. Godette, OncoImmunology, 2018, 7, 1442168 Search PubMed.
- C. Grassberger, S. G. Ellsworth, M. Q. Wilks, F. K. Keane and J. S. Loeffler, Nat. Rev. Clin. Oncol., 2019, 16, 729–745 CrossRef.
- X. Xiao, H. Cai, Q. Huang, B. Wang, X. Wang, Q. Luo, Y. Li, H. Zhang, Q. Gong, X. Ma, Z. Gu and K. Luo, Bioact. Mater., 2023, 19, 538–549 CrossRef CAS PubMed.
- S. Harmsen, E. I. Medine, M. Moroz, F. Nurili, J. Lobo, Y. Dong, M. Turkekul, N. V. K. Pillarsetty, R. Ting, V. Ponomarev, O. Akin and O. Aras, Biomaterials, 2021, 269, 120630 Search PubMed.
- E. Kim and H. Koo, Chem. Sci., 2019, 10, 7835–7851 RSC.
- M. Geppert and M. Himly, Front. Immunol., 2021, 12, 688927 CrossRef CAS PubMed.
- O. Martinez, J. Sosabowski, J. Maher and S. Papa, J. Nucl. Med., 2019, 60, 730–735 CrossRef CAS.
- J. W. Seo, R. Tavaré, L. M. Mahakian, M. T. Silvestrini, S. Tam, E. S. Ingham, F. B. Salazar, A. D. Borowsky, A. M. Wu and K. W. Ferrara, Clin. Cancer Res., 2018, 24, 4976–4987 CrossRef CAS.
- A. Nguyen, A. Ramesh, S. Kumar, D. Nandi, A. Brouillard, A. Wells, L. Pobezinsky, B. Osborne and A. A. Kulkarni, Sci. Adv., 2020, 6, eabc2777 CrossRef CAS PubMed.
- Q. Lecocq, R. M. Awad, Y. De Vlaeminck, W. De Mey, T. Ertveldt, C. Goyvaerts, G. Raes, K. Thielemans, M. Keyaerts, N. Devoogdt and K. Breckpot, J. Nucl. Med., 2021, 62, 1638–1644 CrossRef CAS.
- C. Shi, Z. Zhou, H. Lin and J. Gao, Small Methods, 2021, 5, 2001025 Search PubMed.
- Y. Zhang, Z. Feng, J. Liu, H. Li, Q. Su, J. Zhang, P. Huang, W. Wang and J. Liu, Bioact. Mater., 2022, 16, 359–371 CrossRef CAS PubMed.
- M. Li, Z. Wang, X. Liu, N. Song, Y. Song, X. Shi, J. Liu, J. Liu and Z. Yu, J. Controlled Release, 2021, 340, 35–47 CrossRef CAS PubMed.
- X. Dong, R. Cheng, S. Zhu, H. Liu, R. Zhou, C. Zhang, K. Chen, L. Mei, C. Wang, C. Su, X. Liu, Z. Gu and Y. Zhao, ACS Nano, 2020, 14, 5400–5416 CrossRef CAS PubMed.
- P. Pei, W. Shen, Y. Zhang, Y. Zhang, Z. Qi, H. Zhou, T. Liu, L. Sun and K. Yang, Biomaterials, 2022, 280, 121326 CrossRef CAS.
- W. Sang, Z. Zhang, G. Wang, L. Xie, J. Li, W. Li, H. Tian and Y. Dai, Adv. Funct. Mater., 2022, 32, 2113168 Search PubMed.
- T. Tian, R. Liang, G. Erel-Akbaba, L. Saad, P. J. Obeid, J. Gao, E. A. Chiocca, R. Weissleder and B. A. Tannous, ACS Nano, 2022, 16, 1940–1953 CrossRef CAS PubMed.
- X. Qin, C. Yang, H. Xu, R. Zhang, D. Zhang, J. Tu, Y. Guo, B. Niu, L. Kong and Z. Zhang, Small, 2021, 17, 2103984 CrossRef CAS PubMed.
- Y. Shi and T. Lammers, Acc. Chem. Res., 2019, 52, 1543–1554 CrossRef CAS PubMed.
- A. N. DuRoss, M. J. Neufeld, S. Rana, C. R. Thomas, Jr. and C. Sun, Adv. Drug Delivery Rev., 2019, 144, 35–56 CrossRef CAS PubMed.
- J. Wolfram and M. Ferrari, Nano Today, 2019, 25, 85–98 CrossRef CAS.
- J. H. Myung, M. J. Eblan, J. M. Caster, S. J. Park, M. J. Poellmann, K. Wang, K. A. Tam, S. M. Miller, C. Shen, R. C. Chen, T. Zhang, J. E. Tepper, B. S. Chera, A. Z. Wang and S. Hong, Clin. Cancer Res., 2018, 24, 2539–2547 CrossRef CAS.
- X. Zhu, X. Wang, B. Li, Y. Zhang, Y. Chen, W. Zhang, Y. Wang, W. Zhai, Z. Liu, S. Liu, J. Sun, Z. Chen and Y. Gao, Small, 2022, 18, 2107001 CrossRef CAS.
- E. L. Dane, A. Belessiotis-Richards, C. Backlund, J. Wang, K. Hidaka, L. E. Milling, S. Bhagchandani, M. B. Melo, S. Wu, N. Li, N. Donahue, K. Ni, L. Ma, M. Okaniwa, M. M. Stevens, A. Alexander-Katz and D. J. Irvine, Nat. Mater., 2022, 21, 710–720 Search PubMed.
- Q. Zhang, J. Zhang, J. Song, Y. Liu, X. Ren and Y. Zhao, ACS Nano, 2021, 15, 8001–8038 CrossRef CAS PubMed.
- Z. Wang, K. Zhi, Z. Ding, Y. Sun, S. Li, M. Li, K. Pu and J. Zou, Semin. Cancer Biol., 2021, 69, 77–90 CrossRef CAS.
- K. I. Joo, Y. Jeong, S. M. Hwang, M. Shin, J. Lee, G. Sharma, H. Lee, S. H. Im and H. J. Cha, Biomaterials, 2020, 263, 120380 Search PubMed.
- P. Debie, C. Lafont, M. Defrise, I. Hansen, D. M. van Willigen, F. W. van Leeuwen, R. Gijsbers, M. D'huyvetter, N. Devoogdt and T. Lahoutte, J. Controlled Release, 2020, 317, 34–42 CrossRef CAS PubMed.
- W. Chen, Y. Yuan and X. Jiang, J. Controlled Release, 2020, 328, 395–406 Search PubMed.
- Z. Tian, M. Liu, Y. Zhang and X. Wang, J. Hematol. Oncol., 2021, 14, 75 Search PubMed.
- W. J. Cheng, K. H. Chuang, Y. J. Lo, M. Chen, Y. J. Chen, S. R. Roffler, H. O. Ho, S. Y. Lin and M. T. Sheu, J. Controlled Release, 2022, 344, 235–248 Search PubMed.
- J. Liu, R. Toy, C. Vantucci, P. Pradhan, Z. Zhang, K. M. Kuo, K. P. Kubelick, D. Huo, J. Wen and J. Kim, Nano Lett., 2021, 21, 875–886 CrossRef CAS.
- L. Sun, F. Shen, Z. Xiong, H. Yang, Z. Dong, J. Xiang, Q. Gu, Q. Ji, C. Fan and Z. Liu, J. Am. Chem. Soc., 2022, 144, 7634–7645 Search PubMed.
- X. Huang, J. Z. Williams, R. Chang, Z. Li, C. E. Burnett, R. Hernandez-Lopez, I. Setiady, E. Gai, D. M. Patterson and W. Yu, Nat. Nanotechnol., 2021, 16, 214–223 Search PubMed.
- J. Li, H. Ren and Y. Zhang, Coordin. Chem. Rev., 2022, 455, 214345 CrossRef CAS.
- Z. Liang, Y. Yang, G. Yu, H. Zhu, X. Xia, C. Chen, D. Fu, M. Li, G. Cheng, C. Xue, L. Shi, H. Zeng and B. Sun, Biomaterials, 2021, 275, 120960 CrossRef CAS PubMed.
- J. Lu, Y. Jiao, G. Cao and Z. Liu, Chem. Eng. J., 2021, 420, 129746 CrossRef CAS.
- R. Chai, L. Yu, C. Dong, Y. Yin, S. Wang, Y. Chen and Q. Zhang, Bioact. Mater., 2022, 17, 276–288 CrossRef CAS PubMed.
- P. Pei, T. Liu, W. Shen, Z. Liu and K. Yang, Mater. Horiz., 2021, 8, 1348–1366 RSC.
- T. Karpov, A. Postovalova, D. Akhmetova, A. R. Muslimov, E. Eletskaya, M. V. Zyuzin and A. S. Timin, Chem. Mater., 2022, 34, 6593–6605 Search PubMed.
- X. Hong, X. Zhong, G. Du, Y. Hou, Y. Zhang, Z. Zhang, T. Gong, L. Zhang and X. Sun, Sci. Adv., 2020, 6, eaaz4462 CrossRef CAS.
- P. A. Bielecki, M. E. Lorkowski, W. M. Becicka, P. U. Atukorale, T. J. Moon, Y. Zhang, M. Wiese, G. Covarrubias, S. Ravichandran and E. Karathanasis, Nanoscale Horiz., 2021, 6, 156–167 Search PubMed.
- M. Sun, P. Gu, Y. Yang, L. Yu, Z. Jiang, J. Li, Y. Le, Y. Chen, Q. Ba and H. Wang, J. Immunother. Cancer, 2021, 9, e002508 CrossRef.
- L. Li, M. Zhen, H. Wang, Z. Sun, W. Jia, Z. Zhao, C. Zhou, S. Liu, C. Wang and C. Bai, Nano Lett., 2020, 20, 4487–4496 CrossRef CAS PubMed.
- X. Zhang, C. He, Y. Sun, X. Liu, Y. Chen, C. Chen, R. Yan, T. Fan, T. Yang, Y. Lu, J. Luo, X. Ma and G. Xiang, Acta Pharm. Sin. B, 2021, 11, 3608–3621 Search PubMed.
- X. Guan, L. Sun, Y. Shen, F. Jin, X. Bo, C. Zhu, X. Han, X. Li, Y. Chen, H. Xu and W. Yue, Nat. Commun., 2022, 13, 2834 CrossRef CAS PubMed.
- S. Bonvalot, P. L. Rutkowski, J. Thariat, S. Carrère, A. Ducassou, M. P. Sunyach, P. Agoston, A. Hong, A. Mervoyer, M. Rastrelli, V. Moreno, R. K. Li, B. Tiangco, A. C. Herraez, A. Gronchi, L. Mangel, T. Sy-Ortin, P. Hohenberger, T. de Baère, A. Le Cesne, S. Helfre, E. Saada-Bouzid, A. Borkowska, R. Anghel, A. Co, M. Gebhart, G. Kantor, A. Montero, H. H. Loong, R. Vergés, L. Lapeire, S. Dema, G. Kacso, L. Austen, L. Moureau-Zabotto, V. Servois, E. Wardelmann, P. Terrier, A. J. Lazar, J. Bovée, C. Le Péchoux and Z. Papai, Lancet Oncol., 2019, 20, 1148–1159 CrossRef CAS.
- X. Wang, X. Zhong, J. Li, Z. Liu and L. Cheng, Chem. Soc. Rev., 2021, 50, 8669–8742 RSC.
- K. Lu, C. He, N. Guo, C. Chan, K. Ni, G. Lan, H. Tang, C. Pelizzari, Y. X. Fu, M. T. Spiotto, R. R. Weichselbaum and W. Lin, Nat. Biomed. Eng., 2018, 2, 600–610 CrossRef CAS PubMed.
- K. Ni, T. Luo, G. T. Nash and W. Lin, Acc. Chem. Res., 2020, 53, 1739–1748 CrossRef CAS.
- K. Ni, G. Lan, N. Guo, A. Culbert, T. Luo, T. Wu, R. R. Weichselbaum and W. Lin, Sci. Adv., 2020, 6, eabb5223 CrossRef CAS.
- K. Ni, G. Lan, C. Chan, B. Quigley, K. Lu, T. Aung, N. Guo, P. La Riviere, R. R. Weichselbaum and W. Lin, Nat. Commun., 2018, 9, 2351 CrossRef.
- K. Ni, G. Lan, Y. Song, Z. Hao and W. Lin, Chem. Sci., 2020, 11, 7641–7653 RSC.
- K. Ni, Z. Xu, A. Culbert, T. Luo, N. Guo, K. Yang, E. Pearson, B. Preusser, T. Wu, P. La Riviere, R. R. Weichselbaum, M. T. Spiotto and W. Lin, Nat. Biomed. Eng., 2022, 6, 144–156 CrossRef CAS PubMed.
- G. Lan, K. Ni, S. S. Veroneau, Y. Song and W. Lin, J. Am. Chem. Soc., 2018, 140, 16971–16975 CrossRef CAS PubMed.
- Z. Xu, K. Ni, J. Mao, T. Luo and W. Lin, Adv. Mater., 2021, 33, 2104249 CrossRef CAS.
- R. Li, Y. He, S. Zhang, J. Qin and J. Wang, Acta Pharm. Sin. B, 2018, 8, 14–22 CrossRef PubMed.
- L. Chen, W. Hong, W. Ren, T. Xu, Z. Qian and Z. He, Signal Transduction Targeted Ther., 2021, 6, 225 CrossRef.
- Z. Li, H. Cai, Z. Li, L. Ren, X. Ma, H. Zhu, Q. Gong, H. Zhang, Z. Gu and K. Luo, Bioact. Mater., 2023, 21, 299–312 CrossRef CAS PubMed.
- F. Combes, E. Meyer and N. N. Sanders, J. Controlled Release, 2020, 327, 70–87 CrossRef CAS.
- D. Xia, D. Hang, Y. Li, W. Jiang, J. Zhu, Y. Ding, H. Gu and Y. Hu, ACS Nano, 2020, 14, 15654–15668 CrossRef.
- M. Mehanny, C.-M. Lehr and G. Fuhrmann, Adv. Drug Delivery Rev., 2021, 173, 164–180 CrossRef CAS.
- S. R. Kang, D. H. Nguyen, S. W. Yoo and J. J. Min, Adv. Drug Delivery Rev., 2021, 181, 114085 CrossRef.
- Y. Zeng, S. Li, S. Zhang, L. Wang, H. Yuan and F. Hu, Acta Pharm. Sin. B, 2022, 12, 3233–3254 CrossRef CAS PubMed.
- S. K. Alsaiari, S. S. Qutub, S. Sun, W. Baslyman, M. Aldehaiman, M. Alyami, A. Almalik, R. Halwani, J. Merzaban and Z. Mao, Sci. Adv., 2021, 7, eabe7174 CrossRef CAS PubMed.
- L. Gong, Y. Zhang, J. Zhao, Y. Zhang, K. Tu, L. Jiao, Q. Xu, M. Zhang and S. Han, Small, 2022, 18, 2107656 CrossRef CAS.
- G. Pang, C. Chen, Y. Liu, T. Jiang, H. Yu, Y. Wu, Y. Wang, F. J. Wang, Z. Liu and L. W. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 42661–42670 CrossRef CAS PubMed.
- X. Han, S. Shen, Q. Fan, G. Chen, E. Archibong, G. Dotti, Z. Liu, Z. Gu and C. Wang, Sci. Adv., 2019, 5, eaaw6870 CrossRef CAS.
- C. Gong, X. Yu, W. Zhang, L. Han, R. Wang, Y. Wang, S. Gao and Y. Yuan, J. Nanobiotechnol., 2021, 19, 58 CrossRef CAS.
- M. Z. Zou, Z. H. Li, X. F. Bai, C. J. Liu and X. Z. Zhang, Nano Lett., 2021, 21, 8609–8618 CrossRef CAS PubMed.
- M. Hu, J. Zhang, L. Kong, Y. Yu, Q. Hu, T. Yang, Y. Wang, K. Tu, Q. Qiao, X. Qin and Z. Zhang, ACS Nano, 2021, 15, 3123–3138 CrossRef CAS.
- J. H. Park, Y. Jiang, J. Zhou, H. Gong, A. Mohapatra, J. Heo, W. Gao, R. H. Fang and L. Zhang, Sci. Adv., 2021, 7, eabf7820 CrossRef CAS PubMed.
- D. X. Zhang, L. T. Vu, N. N. Ismail, M. T. N. Le and A. Grimson, Semin. Cancer Biol., 2021, 74, 24–44 CrossRef CAS.
- Y. Guo, G. Hu, Y. Xia, H. Li, J. Yuan, J. Zhang, Y. Chen, H. Guo, Y. Yang, Y. Wang and Z. Deng, Bioact. Mater., 2022, 16, 204–217 CrossRef CAS PubMed.
- Z. Quinn, W. Mao, Y. Xia, R. John and Y. Wan, Bioact. Mater., 2021, 6, 749–756 CrossRef CAS PubMed.
- M. D'Huyvetter, J. Vos, V. Caveliers, I. Vaneycken, J. Heemskerk, F. P. Duhoux, C. Fontaine, M. Vanhoeij, A. D. Windhorst, F. V. Aa, N. H. Hendrikse, J. L. E. Eersels, H. Everaert, P. Gykiere, N. Devoogdt, G. Raes, T. Lahoutte and M. Keyaerts, J. Nucl. Med., 2021, 62, 1097–1105 CrossRef.
- J. Garousi, E. von Witting, J. Borin, A. Vorobyeva, M. Altai, O. Vorontsova, M. W. Konijnenberg, M. Oroujeni, A. Orlova, V. Tolmachev and S. Hober, Biomaterials, 2021, 266, 120381 CrossRef CAS PubMed.
- M. E. Kieffer, A. M. Patel, S. A. Hollingsworth and W. M. Seganish, Expert Opin. Ther. Pat., 2020, 30, 825–845 CrossRef CAS.
- Y. Y. Janjigian, A. Kawazoe, P. Yañez, N. Li, S. Lonardi, O. Kolesnik, O. Barajas, Y. Bai, L. Shen, Y. Tang, L. S. Wyrwicz, J. Xu, K. Shitara, S. Qin, E. Van Cutsem, J. Tabernero, L. Li, S. Shah, P. Bhagia and H. C. Chung, Nature, 2021, 600, 727–730 CrossRef CAS PubMed.
- Z. Tang, Y. Xiao, N. Kong, C. Liu, W. Chen, X. Huang, D. Xu, J. Ouyang, C. Feng, C. Wang, J. Wang, H. Zhang and W. Tao, Acta Pharm. Sin. B, 2021, 11, 3447–3464 CrossRef CAS PubMed.
- G. Peng, T. Duan, M. Guo, Y. Xue, C. Chen, Y. Li, K. Leifer and B. Fadeel, Nanoscale, 2021, 13, 13072–13084 RSC.
- M. Guo, J. Liu, X. Chen, Z. You, F. Gao, T. Liu, J. Ren, J. Liu, Z. Xiong and Y. Liu, Nano Today, 2022, 45, 101543 CrossRef CAS.
- D. Huo, X. Jiang and Y. Hu, Adv. Mater., 2020, 32, 1904337 CAS.
- L. Jia, X. Li, H. Liu, J. Xia, X. Shi and M. Shen, Nano Today, 2021, 36, 101022 CrossRef CAS.
- J. Li, X. Jiang, H. Li, M. Gelinsky and Z. Gu, Adv. Mater., 2021, 33, 2004172 CrossRef CAS.
- Y. Wang, J. Wang, D. Zhu, Y. Wang, G. Qing, Y. Zhang, X. Liu and X. J. Liang, Acta Pharm. Sin. B, 2021, 11, 886–902 CrossRef CAS PubMed.
- T. Song, Y. Xia, Y. Du, M. W. Chen, H. Qing and G. Ma, Adv. Mater., 2021, 33, 2100106 CrossRef CAS PubMed.
- V. T. Cong, W. Wang, R. D. Tilley, G. Sharbeen, P. A. Phillips, K. Gaus and J. J. Gooding, Adv. Funct. Mater., 2021, 31, 2007880 CrossRef CAS.
- M. Zhang, X. Chen, C. Li and X. Shen, J. Controlled Release, 2020, 319, 46–62 CrossRef CAS PubMed.
- J. W. Breslin, Y. Yang, J. P. Scallan, R. S. Sweat, S. P. Adderley and W. L. Murfee, Compr. Physiol., 2018, 9, 207–299 Search PubMed.
- Y. Yang, J. Xu, Y. Sun, L. Mo, B. Liu, X. Pan, Z. Liu and W. Tan, J. Am. Chem. Soc., 2021, 143, 8391–8401 CrossRef CAS.
- Y. Kim, H. J. Jung, Y. Lee, S. Koo, R. Thangam, W. Y. Jang, S. Y. Kim, S. Park, S. Lee, G. Bae, K. D. Patel, Q. Wei, K. B. Lee, R. Paulmurugan, W. K. Jeong, T. Hyeon, D. Kim and H. Kang, J. Am. Chem. Soc., 2022, 144, 5769–5783 CrossRef CAS.
- R. Sharma, K. Liaw, A. Sharma, A. Jimenez, M. Chang, S. Salazar, I. Amlani, S. Kannan and R. M. Kannan, J. Controlled Release, 2021, 337, 179–192 Search PubMed.
- A. E. Barberio, S. G. Smith, S. Correa, C. Nguyen, B. Nhan, M. Melo, T. Tokatlian, H. Suh, D. J. Irvine and P. T. Hammond, ACS Nano, 2020, 14, 11238–11253 CrossRef CAS.
- W. Yang, H. Deng, S. Zhu, J. Lau, R. Tian, S. Wang, Z. Zhou, G. Yu, L. Rao, L. He, Y. Ma and X. Chen, Sci. Adv., 2020, 6, eabd1631 CrossRef CAS.
- Y. Wang, S. Li, X. Wang, Q. Chen, Z. He, C. Luo and J. Sun, Biomaterials, 2021, 271, 120737 CrossRef CAS.
- B. Q. Chen, Y. Zhao, Y. Zhang, Y. J. Pan, H. Y. Xia, R. K. Kankala, S. B. Wang, G. Liu and A. Z. Chen, Bioact. Mater., 2023, 21, 1–19 CrossRef CAS.
- Q. Chen, M. Chen and Z. Liu, Chem. Soc. Rev., 2019, 48, 5506–5526 RSC.
- G. Kelly, J. J. Milligan, E. M. Mastria, S. Kim, S. R. Zelenetz, J. Dobbins, L. Y. Cai, X. Li, S. K. Nair and A. Chilkoti, J. Controlled Release, 2022, 343, 267–276 CrossRef CAS PubMed.
- J. Xu, R. Saklatvala, S. Mittal, S. Deshmukh and A. Procopio, Adv. Sci., 2020, 7, 1903394 CrossRef CAS PubMed.
- A. B. Cook and P. Decuzzi, ACS Nano, 2021, 15, 2068–2098 CrossRef CAS PubMed.
- C. Zhang and K. Pu, Chem. Soc. Rev., 2020, 49, 4234–4253 RSC.
- H. Ding, P. Tan, S. Fu, X. Tian, H. Zhang, X. Ma, Z. Gu and K. Luo, J. Controlled Release, 2022, 348, 206–238 CrossRef CAS.
- M. Liu, L. Huang, W. Zhang, X. Wang, Y. Geng, Y. Zhang, L. Wang, W. Zhang, Y. J. Zhang, S. Xiao, Y. Bao, M. Xiong and J. Wang, Nat. Nanotechnol., 2022, 17, 541–551 CrossRef CAS.
- Y. Li, M. Jiang, Z. Deng, S. Zeng and J. Hao, Adv. Sci., 2021, 8, 2004391 Search PubMed.
- R. Misra, K. Sarkar, J. Lee, V. J. Pizzuti, D. S. Lee, M. P. Currie, S. E. Torregrosa-Allen, D. E. Long, G. A. Durm and M. P. Langer, J. Controlled Release, 2019, 303, 237–252 Search PubMed.
- W. Fan, W. Tang, J. Lau, Z. Shen, J. Xie, J. Shi and X. Chen, Adv. Mater., 2019, 31, 1806381 Search PubMed.
- T. Li, S. Pan, S. Gao, W. Xiang, C. Sun, W. Cao and H. Xu, Angew. Chem., Int. Ed., 2020, 59, 2700–2704 CrossRef CAS PubMed.
- Y. Yu, Z. Feng, J. Liu, X. Hou, X. Zhou, J. Gao, W. Wang, Y. Zhang, G. Li and J. Liu, ACS Omega, 2021, 6, 19445–19457 CrossRef CAS.
- L. Zhang, W. Wang and S. Wang, Expert Rev. Vaccines, 2015, 14, 1509–1523 Search PubMed.
- A. Marabelle, R. Andtbacka, K. Harrington, I. Melero, R. Leidner, T. de Baere, C. Robert, P. A. Ascierto, J.-F. Baurain and M. Imperiale, Ann. Oncol., 2018, 29, 2163–2174 CrossRef CAS PubMed.
- O. M. Feeney, D. H. Brundel, N. L. Trevaskis, E. Cao, L. M. Kaminskas and C. J. Porter, Adv. Drug Delivery Rev., 2020, 160, 115–135 Search PubMed.
- L. Zhang, X. Sun, Y. Jia, X. Liu, M. Dong, Z. P. Xu and R. Liu, Nano Today, 2020, 35, 100923 CrossRef CAS.
- Y. Wang, W. Deng, N. Li, S. Neri, A. Sharma, W. Jiang and S. H. Lin, Front. Pharmacol., 2018, 9, 185 CrossRef.
- S. Ghosh, A. Javia, S. Shetty, D. Bardoliwala, K. Maiti, S. Banerjee, A. Khopade, A. Misra, K. Sawant and S. Bhowmick, J. Controlled Release, 2021, 337, 27–58 CrossRef CAS.
- A. Bassez, H. Vos, L. Van Dyck, G. Floris, I. Arijs, C. Desmedt, B. Boeckx, M. V. Bempt, I. Nevelsteen and K. Lambein, Nat. Med., 2021, 27, 820–832 Search PubMed.
- H. Jung, H. S. Kim, J. Y. Kim, J. M. Sun, J. S. Ahn, M. J. Ahn, K. Park, M. Esteller, S. H. Lee and J. K. Choi, Nat. Commun., 2019, 10, 4278 CrossRef.
- H. Lam, L. K. McNeil, H. Starobinets, V. L. DeVault, R. B. Cohen, P. Twardowski, M. L. Johnson, M. L. Gillison, M. N. Stein, U. N. Vaishampayan, A. P. DeCillis, J. J. Foti, V. Vemulapalli, E. Tjon, K. Ferber, D. B. DeOliveira, W. Broom, P. Agnihotri, E. M. Jaffee, K. K. Wong, C. G. Drake, P. M. Carroll, T. A. Davis and J. B. Flechtner, Cancer Discovery, 2021, 11, 696–713 CrossRef CAS PubMed.
- M. Gromeier, M. C. Brown, G. Zhang, X. Lin, Y. Chen, Z. Wei, N. Beaubier, H. Yan, Y. He, A. Desjardins, J. E. Herndon, 2nd, F. S. Varn, R. G. Verhaak, J. Zhao, D. P. Bolognesi, A. H. Friedman, H. S. Friedman, F. McSherry, A. M. Muscat, E. S. Lipp, S. K. Nair, M. Khasraw, K. B. Peters, D. Randazzo, J. H. Sampson, R. E. McLendon, D. D. Bigner and D. M. Ashley, Nat. Commun., 2021, 12, 352 Search PubMed.
- M. V. Guerin, V. Finisguerra, B. J. Van den Eynde, N. Bercovici and A. Trautmann, Elife, 2020, 9, e50740 Search PubMed.
- E. Schültke, S. Bayat, S. Bartzsch, E. Bräuer-Krisch, V. Djonov, S. Fiedler, C. Fernandez-Palomo, F. Jaekel, P. Pellicioli, V. Trappetti and G. Hildebrandt, Int. J. Radiat. Oncol. Biol. Phys., 2021, 110, 521–525 CrossRef.
- H. Watanabe, K. Numata, T. Ito, K. Takagi and A. Matsukawa, Shock, 2004, 22, 460–466 CrossRef CAS.
- S. Zaaijer, S. C. Groen and N. E. Sanjana, Nat. Biotechnol., 2021, 39, 666–670 CrossRef CAS PubMed.
- N. Tateshita, N. Miura, H. Tanaka, T. Masuda, S. Ohtsuki, K. Tange, Y. Nakai, H. Yoshioka and H. Akita, J. Controlled Release, 2019, 310, 36–46 Search PubMed.
- Z. Zhang, L. Liu, C. Ma, X. Cui, R. H. W. Lam and W. Chen, Small Methods, 2021, 5, 2100197 CrossRef CAS PubMed.
- P. De La Rochere, S. Guil-Luna, D. Decaudin, G. Azar, S. S. Sidhu and E. Piaggio, Trends Immunol., 2018, 39, 748–763 Search PubMed.
- C. Peres, A. I. Matos, L. I. F. Moura, R. C. Acúrcio, B. Carreira, S. Pozzi, D. Vaskovich-Koubi, R. Kleiner, R. Satchi-Fainaro and H. F. Florindo, Adv. Drug Delivery Rev., 2021, 172, 148–182 Search PubMed.
- H.-Y. Kim, R. Li, T. S. Ng, G. Courties, C. B. Rodell, M. Prytyskach, R. H. Kohler, M. J. Pittet, M. Nahrendorf and R. Weissleder, ACS Nano, 2018, 12, 12015–12029 Search PubMed.
- G. Lv, X. Sun, L. Qiu, Y. Sun, K. Li, Q. Liu, Q. Zhao, S. Qin and J. Lin, J. Nucl. Med., 2020, 61, 117–122 CrossRef CAS.
- Z. Zhou, H. Deng, W. Yang, Z. Wang, L. Lin, J. Munasinghe, O. Jacobson, Y. Liu, L. Tang, Q. Ni, F. Kang, Y. Liu, G. Niu, R. Bai, C. Qian, J. Song and X. Chen, Nat. Commun., 2020, 11, 3032 CrossRef CAS PubMed.
- H. Yu, H. Guo, H. Zu, H. Ding, S. Lin, Y. Wang, L. W. Zhang and Y. Wang, Aggregate, 2022, 3, 194 Search PubMed.
- Y. Wang, J. Chen, R. Duan, R. Gu, W. Wang, J. Wu, H. Lian, Y. Hu and A. Yuan, Adv. Mater., 2022, 34, 2109726 Search PubMed.
- Z. Huang, Y. Wang, D. Yao, J. Wu, Y. Hu and A. Yuan, Nat. Commun., 2021, 12, 145 Search PubMed.
- Y. Yang, B. Liu, Y. Liu, J. Chen, Y. Sun, X. Pan, J. Xu, S. Xu, Z. Liu and W. Tan, Nano Lett., 2022, 22, 2826–2834 Search PubMed.
- X. Zhu, X. Wang, B. Li, Y. Zhang, Y. Chen, W. Zhang, Y. Wang, W. Zhai, Z. Liu, S. Liu, J. Sun, Z. Chen and Y. Gao, Small, 2022, 18, 2107001 Search PubMed.
- Y. Chen, S. Liu, P. Gao, W. Pan, M. Shi, J. Wang, N. Li and B. Tang, Sci. China: Mater., 2022 DOI:10.1007/s40843-022-2140-7.
- W. J. Smith, G. Wang, H. Gaikwad, V. P. Vu, E. Groman, D. W. Bourne and D. Simberg, ACS Nano, 2018, 12, 12523–12532 CrossRef CAS.
- S. Jia, S. Dong, H. Liu, H. Yu, Z. Chen, S. Wang, W. Li, R. Peng, F. Li, Q. Jiang and J. Liu, Biomater. Sci., 2022, 10, 3309–3322 RSC.
- H. K. Angell, D. Bruni, J. C. Barrett, R. Herbst and J. Galon, Clin. Cancer Res., 2020, 26, 332–339 CrossRef CAS.
- T. S. C. Ng, V. Gunda, R. Li, M. Prytyskach, Y. Iwamoto, R. H. Kohler, S. Parangi, R. Weissleder and M. A. Miller, Radiology, 2021, 298, 123–132 Search PubMed.
- R. Tian, C. Ke, L. Rao, J. Lau and X. Chen, Adv. Drug Delivery Rev., 2020, 161–162, 145–160 CrossRef CAS PubMed.
- W. Wei, Z. T. Rosenkrans, J. Liu, G. Huang, Q. Y. Luo and W. Cai, Chem. Rev., 2020, 120, 3787–3851 CrossRef CAS PubMed.
- K. Herrmann, M. Schwaiger, J. S. Lewis, S. B. Solomon, B. J. McNeil, M. Baumann, S. S. Gambhir, H. Hricak and R. Weissleder, Lancet Oncol., 2020, 21, 146–156 Search PubMed.
- Y. Zhao, T. Zhang, Y. Wang, D. Lu, J. Du, X. Feng, H. Zhou, N. Liu, H. Zhu and S. Qin, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2010333118 CrossRef CAS PubMed.
- R. Cristescu, R. Mogg, M. Ayers, A. Albright, E. Murphy, J. Yearley, X. Sher, X. Q. Liu, H. Lu and M. Nebozhyn, Science, 2018, 362, eaar3593 CrossRef.
- T. A. Chan, M. Yarchoan, E. Jaffee, C. Swanton, S. A. Quezada, A. Stenzinger and S. Peters, Ann. Oncol., 2019, 30, 44–56 Search PubMed.
- C. Valero, M. Lee, D. Hoen, J. Wang, Z. Nadeem, N. Patel, M. A. Postow, A. N. Shoushtari, G. Plitas and V. P. Balachandran, Nat. Genet., 2021, 53, 11–15 CrossRef CAS PubMed.
- H. F. Huang, H. Zhu, G. H. Li, Q. Xie, X. T. Yang, X. X. Xu, X. B. Tian, Y. K. Wan and Z. Yang, Bioconjugate Chem., 2019, 30, 2614–2623 Search PubMed.
- W. Wei, Q. Liu, D. Jiang, H. Zhao, C. J. Kutyreff, J. W. Engle, J. Liu and W. Cai, Adv. Sci., 2020, 7, 1903595 CrossRef CAS.
- I. H. Song, M. S. Jeong, H. J. Hong, J. I. Shin, Y. S. Park, S.-K. Woo, B. S. Moon, K. I. Kim, Y. J. Lee and J. H. Kang, Clin. Cancer Res., 2019, 25, 6148–6159 CrossRef CAS PubMed.
- A. Bagaev, N. Kotlov, K. Nomie, V. Svekolkin, A. Gafurov, O. Isaeva, N. Osokin, I. Kozlov, F. Frenkel and O. Gancharova, Cancer Cell, 2021, 39, 845–865 Search PubMed.
- Y. Zhang and L. Chen, JAMA Oncol., 2016, 2, 1403–1404 CrossRef PubMed.
- E. Borcoman, Y. Kanjanapan, S. Champiat, S. Kato, V. Servois, R. Kurzrock, S. Goel, P. Bedard and C. Le Tourneau, Ann. Oncol., 2019, 30, 385–396 CrossRef CAS PubMed.
- Q. Fu, H. Feng, L. Su, X. Zhang, L. Liu, F. Fu, H. Yang and J. Song, Angew. Chem., Int. Ed., 2022, 61, 202112237 Search PubMed.
- S. B. Goldberg, A. Narayan, A. J. Kole, R. H. Decker, J. Teysir, N. J. Carriero, A. Lee, R. Nemati, S. K. Nath, S. M. Mane, Y. Deng, N. Sukumar, D. Zelterman, D. J. Boffa, K. Politi, S. N. Gettinger, L. D. Wilson, R. S. Herbst and A. A. Patel, Clin. Cancer Res., 2018, 24, 1872–1880 CrossRef CAS.
- E. Sanz-Garcia, E. Zhao, S. V. Bratman and L. L. Siu, Sci. Adv., 2022, 8, eabi8618 CrossRef CAS PubMed.
- Z. Dong, X. Xue, H. Liang, J. Guan and L. Chang, Anal. Chem., 2021, 93, 1855–1865 CrossRef CAS PubMed.
- G. De Rubis, S. Rajeev Krishnan and M. Bebawy, Trends Pharmacol. Sci., 2019, 40, 172–186 CrossRef CAS.
- Y. Liu, J. Zugazagoitia, F. S. Ahmed, B. S. Henick, S. N. Gettinger, R. S. Herbst, K. A. Schalper and D. L. Rimm, Clin. Cancer Res., 2020, 26, 970–977 CrossRef CAS PubMed.
- F. Bensch, E. L. van der Veen, M. N. Lub-de Hooge, A. Jorritsma-Smit, R. Boellaard, I. C. Kok, S. F. Oosting, C. P. Schröder, T. J. N. Hiltermann, A. J. van der Wekken, H. J. M. Groen, T. C. Kwee, S. G. Elias, J. A. Gietema, S. S. Bohorquez, A. de Crespigny, S. P. Williams, C. Mancao, A. H. Brouwers, B. M. Fine and E. G. E. de Vries, Nat. Med., 2018, 24, 1852–1858 CrossRef CAS.
- E. B. Ehlerding, H. J. Lee, T. E. Barnhart, D. Jiang, L. Kang, D. G. McNeel, J. W. Engle and W. Cai, Bioconjugate Chem., 2019, 30, 1434–1441 CrossRef CAS.
- L. Tian, M. Shao, Y. Gong, Y. Chao, T. Wei, K. Yang, Q. Chen and Z. Liu, Sci. China: Chem., 2022, 65, 574–583 Search PubMed.
- Y. C. Ou, X. Wen, C. A. Johnson, D. Shae, O. D. Ayala, J. A. Webb, E. C. Lin, R. C. DeLapp, K. L. Boyd, A. Richmond, A. Mahadevan-Jansen, M. Rafat, J. T. Wilson, J. M. Balko, M. N. Tantawy, A. E. Vilgelm and R. Bardhan, ACS Nano, 2020, 14, 651–663 Search PubMed.
- L. Devkota, Z. Starosolski, C. H. Rivas, I. Stupin, A. Annapragada, K. B. Ghaghada and R. Parihar, Sci. Adv., 2020, 6, eaba6156 Search PubMed.
- T. Li, D. Chen, H. Liu, Y. Tao, X. He, S. Zang, J. Li, L. Zhang, M. Li, J. Liu and Q. He, Nanoscale, 2022, 14, 13098–13112 RSC.
- G. Delfanti, F. Cortesi, A. Perini, G. Antonini, L. Azzimonti, C. de Lalla, C. Garavaglia, M. L. Squadrito, M. Fedeli, M. Consonni, S. Sesana, F. Re, H. Shen, P. Dellabona and G. Casorati, Sci. Immunol., 2022, 7, eabn6563 Search PubMed.
- E. Loeuillard, J. Yang, E. Buckarma, J. Wang, Y. Liu, C. Conboy, K. D. Pavelko, Y. Li, D. O'Brien, C. Wang, R. P. Graham, R. L. Smoot, H. Dong and S. Ilyas, J. Clin. Invest., 2020, 130, 5380–5396 Search PubMed.
- H. J. Park, K. W. Kim, J. Pyo, C. H. Suh, S. Yoon, H. Hatabu and M. Nishino, Radiology, 2020, 297, 87–96 Search PubMed.
- L. B. Costa, M. A. Queiroz, F. G. Barbosa, R. F. Nunes, E. C. Zaniboni, M. M. Ruiz, D. Jardim, J. F. Gomes Marin, G. G. Cerri and C. A. Buchpiguel, Radiographics, 2021, 41, 120–143 Search PubMed.
- B. Kas, H. Talbot, R. Ferrara, C. Richard, J. P. Lamarque, S. Pitre-Champagnat, D. Planchard, C. Balleyguier, B. Besse, L. Mezquita, N. Lassau and C. Caramella, JAMA Oncol., 2020, 6, 1039–1046 Search PubMed.
- M. R. Sheen and S. Fiering, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2019, 11, e1524 Search PubMed.
- L. Qin, H. Zhang, Y. Zhou, C. S. Umeshappa and H. Gao, Small, 2021, 17, 2006000 CrossRef CAS PubMed.
- W. H. Li and Y. M. Li, Chem. Rev., 2020, 120, 11420–11478 Search PubMed.
- S. Cuzzubbo, S. Mangsbo, D. Nagarajan, K. Habra, A. G. Pockley and S. E. B. McArdle, Front. Immunol., 2020, 11, 615240 CrossRef CAS.
- E. B. Golden, A. E. Marciscano and S. C. Formenti, Int. J. Radiat. Oncol. Biol. Phys., 2020, 108, 891–898 Search PubMed.
- P. H. Lizotte, A. M. Wen, M. R. Sheen, J. Fields, P. Rojanasopondist, N. F. Steinmetz and S. Fiering, Nat. Nanotechnol., 2016, 11, 295–303 CrossRef CAS.
- M. Fusciello, F. Fontana, S. Tähtinen, C. Capasso, S. Feola, B. Martins, J. Chiaro, K. Peltonen, L. Ylösmäki, E. Ylösmäki, F. Hamdan, O. K. Kari, J. Ndika, H. Alenius, A. Urtti, J. T. Hirvonen, H. A. Santos and V. Cerullo, Nat. Commun., 2019, 10, 5747 Search PubMed.
- N. Gong, Y. Zhang, X. Teng, Y. Wang, S. Huo, G. Qing, Q. Ni, X. Li, J. Wang, X. Ye, T. Zhang, S. Chen, Y. Wang, J. Yu, P. C. Wang, Y. Gan, J. Zhang, M. J. Mitchell, J. Li and X. J. Liang, Nat. Nanotechnol., 2020, 15, 1053–1064 CrossRef CAS.
- J. Xu, J. Lv, Q. Zhuang, Z. Yang, Z. Cao, L. Xu, P. Pei, C. Wang, H. Wu, Z. Dong, Y. Chao, C. Wang, K. Yang, R. Peng, Y. Cheng and Z. Liu, Nat. Nanotechnol., 2020, 15, 1043–1052 CrossRef CAS.
- X. Xiong, J. Zhao, J. Pan, C. Liu, X. Guo and S. Zhou, Nano Lett., 2021, 21, 8418–8425 Search PubMed.
- M. Saxena, S. H. van der Burg, C. J. M. Melief and N. Bhardwaj, Nat. Rev. Cancer, 2021, 21, 360–378 Search PubMed.
- M. Luo, Z. Liu, X. Zhang, C. Han, L. Z. Samandi, C. Dong, B. D. Sumer, J. Lea, Y. X. Fu and J. Gao, J. Controlled Release, 2019, 300, 154–160 Search PubMed.
- J. Wilhelm, M. Quiñones-Pérez, J. Wang, X. Wang, V. S. Basava and J. Gao, J. Controlled Release, 2021, 329, 353–360 CrossRef CAS.
- R. B. Patel, M. Ye, P. M. Carlson, A. Jaquish, L. Zangl, B. Ma, Y. Wang, I. Arthur, R. Xie, R. J. Brown, X. Wang, R. Sriramaneni, K. Kim, S. Gong and Z. S. Morris, Adv. Mater., 2019, 31, 1902626 CrossRef CAS.
- X. Li, X. Wang, A. Ito and N. M. Tsuji, Nat. Commun., 2020, 11, 3858 CrossRef.
- H. Zhao, J. Xu, Y. Li, X. Guan, X. Han, Y. Xu, H. Zhou, R. Peng, J. Wang and Z. Liu, ACS Nano, 2019, 13, 13127–13135 CrossRef CAS.
- C. Wan, Y. Sun, Y. Tian, L. Lu, X. Dai, J. Meng, J. Huang, Q. He, B. Wu, Z. Zhang, K. Jiang, D. Hu, G. Wu, J. F. Lovell, H. Jin and K. Yang, Sci. Adv., 2020, 6, eaay9789 CrossRef CAS.
- B. Pineda, F. J. Sánchez García, N. K. Olascoaga, V. Pérez de la Cruz, A. Salazar, S. Moreno-Jiménez, N. Hernández Pedro, A. Márquez-Navarro, A. Ortiz Plata and J. Sotelo, Mol. Ther., 2019, 27, 1612–1620 CrossRef CAS.
- Y. Chao, L. Xu, C. Liang, L. Feng, J. Xu, Z. Dong, L. Tian, X. Yi, K. Yang and Z. Liu, Nat. Biomed. Eng., 2018, 2, 611–621 Search PubMed.
- J. Zhang, M. Yang, X. Fan, M. Zhu, Y. Yin, H. Li, J. Chen, S. Qin, H. Zhang, K. Zhang and F. Yu, J. Nanobiotechnol., 2022, 20, 103 CrossRef CAS.
- G. Kroemer, L. Galluzzi, O. Kepp and L. Zitvogel, Annu. Rev. Immunol., 2013, 31, 51–72 Search PubMed.
- Z. Li, X. Lai, S. Fu, L. Ren, H. Cai, H. Zhang, Z. Gu, X. Ma and K. Luo, Adv. Sci., 2022, 9, 2201734 CrossRef CAS.
- Q. Li, C. Chen, J. Kong, L. Li, J. Li and Y. Huang, Acta Pharm. Sin. B, 2022, 12, 2533–2549 CrossRef CAS.
- R. Mezzapelle, M. E. Bianchi and M. P. Crippa, Cell Death Dis., 2021, 12, 869 CrossRef.
- A. Banstola, K. Poudel, J. O. Kim, J. H. Jeong and S. Yook, J. Controlled Release, 2021, 337, 505–520 CrossRef CAS.
- D. R. Green, Cell, 2019, 177, 1094–1107 Search PubMed.
- S. Sen, M. Won, M. S. Levine, Y. Noh, A. C. Sedgwick, J. S. Kim, J. L. Sessler and J. F. Arambula, Chem. Soc. Rev., 2022, 51, 1212–1233 RSC.
- A. D. Garg, L. Vandenberk, C. Koks, T. Verschuere, L. Boon, S. W. Van Gool and P. Agostinis, Sci. Transl. Med., 2016, 8, 328ra27 Search PubMed.
- M. Z. Jin and X. P. Wang, Front. Immunol., 2021, 12, 697964 CrossRef.
- J. Li, Y. Luo and K. Pu, Angew. Chem., Int. Ed., 2021, 60, 12682–12705 CrossRef CAS.
- J. Li, Y. Luo, Z. Zeng, D. Cui, J. Huang, C. Xu, L. Li, K. Pu and R. Zhang, Nat. Commun., 2022, 13, 4032 CrossRef CAS PubMed.
- C. Zhang, Z. Zeng, D. Cui, S. He, Y. Jiang, J. Li, J. Huang and K. Pu, Nat. Commun., 2021, 12, 2934 CrossRef CAS.
- S. Son, J. Kim, J. Kim, B. Kim, J. Lee, Y. Kim, M. Li, H. Kang and J. S. Kim, Chem. Soc. Rev., 2022, 51, 8201–8215 RSC.
- P. Pei, T. Liu, W. Shen, Z. Liu and K. Yang, Mater. Horiz., 2021, 8, 1348–1366 RSC.
- B. A. McDonald, S. Vedam, J. Yang, J. Wang, P. Castillo, B. Lee, A. Sobremonte, S. Ahmed, Y. Ding, A. S. R. Mohamed, P. Balter, N. Hughes, D. Thorwarth, M. Nachbar, M. E. P. Philippens, C. H. J. Terhaard, D. Zips, S. Böke, M. J. Awan, J. Christodouleas and C. D. Fuller, Int. J. Radiat. Oncol. Biol. Phys., 2021, 109, 1606–1618 CrossRef PubMed.
- P. Keall, D. T. Nguyen, R. O'Brien, E. Hewson, H. Ball, P. Poulsen, J. Booth, P. Greer, P. Hunter, L. Wilton, R. Bromley, J. Kipritidis, T. Eade, A. Kneebone, G. Hruby, T. Moodie, A. Hayden, S. Turner, S. Arumugam, M. Sidhom, N. Hardcastle, S. Siva, K. H. Tai, V. Gebski and J. Martin, Int. J. Radiat. Oncol. Biol. Phys., 2020, 107, 530–538 CrossRef.
- M. Zhu, M. Yang, J. Zhang, Y. Yin, X. Fan, Y. Zhang, S. Qin, H. Zhang and F. Yu, Front. Immunol., 2021, 12, 705361 CrossRef CAS PubMed.
- H. K. Birdi, A. Jirovec, S. Cortés-Kaplan, J. Werier, C. Nessim, J. S. Diallo and M. Ardolino, J. Immunother. Cancer, 2021, 9, e001580 Search PubMed.
- L. Xie, G. Wang, W. Sang, J. Li, Z. Zhang, W. Li, J. Yan, Q. Zhao and Y. Dai, Biomaterials, 2021, 269, 120638 Search PubMed.
- Y. Wang, N. Shen, Y. Wang, M. Li, W. Zhang, L. Fan, L. Liu, Z. Tang and X. Chen, Biomater. Sci., 2021, 9, 3019–3027 Search PubMed.
- G. Hou, J. Qian, M. Guo, W. Xu, J. Wang, Y. Wang and A. Suo, Chem. Eng. J., 2022, 435, 134778 CrossRef CAS.
- Z. Huang, D. Yao, Q. Ye, H. Jiang, R. Gu, C. Ji, J. Wu, Y. Hu and A. Yuan, ACS Nano, 2021, 15, 8450–8465 Search PubMed.
- Y. Wang, Y. Ding, D. Yao, H. Dong, C. Ji, J. Wu, Y. Hu and A. Yuan, Small, 2021, 17, 2006231 CrossRef CAS PubMed.
- J. R. Cubillos-Ruiz, S. E. Bettigole and L. H. Glimcher, Cell, 2017, 168, 692–706 Search PubMed.
- B. Choi, H. Choi, B. Yu and D. H. Kim, ACS Nano, 2020, 14, 13115–13126 Search PubMed.
- B. V. Lima, M. J. Oliveira, M. A. Barbosa, R. M. Gonçalves and F. Castro, Mater. Today Adv., 2022, 15, 100252 Search PubMed.
- H. Yu, Y. Yang, T. Jiang, X. Zhang, Y. Zhao, G. Pang, Y. Feng, S. Zhang, F. Wang, Y. Wang, Y. Wang and L. W. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 27536–27547 CrossRef CAS PubMed.
- X. Liu, D. Wang, P. Zhang and Y. Li, Nano Today, 2019, 29, 100801 Search PubMed.
- S. Nizzero, H. Shen, M. Ferrari and B. Corradetti, Trends Cancer, 2020, 6, 40–48 Search PubMed.
- H. Wang, Y. Chao, H. Zhao, X. Zhou, F. Zhang, Z. Zhang, Z. Li, J. Pan, J. Wang, Q. Chen and Z. Liu, ACS Nano, 2022, 16, 664–674 Search PubMed.
- T. R. Cox, Nat. Rev. Cancer, 2021, 21, 217–238 Search PubMed.
- A. K. Pearce and R. K. O'Reilly, Bioconjugate Chem., 2019, 30, 2300–2311 CrossRef CAS PubMed.
- P. Mi, H. Cabral and K. Kataoka, Adv. Mater., 2020, 32, 1902604 CrossRef CAS.
- L. Meng, C. Wang, Y. Lu, G. Sheng, L. Yang, Z. Wu, H. Xu, C. Han, Y. Lu and F. Han, ACS Appl. Mater. Interfaces, 2021, 13, 11657–11671 CrossRef CAS.
- J. Wei, D. Wu, Y. Shao, B. Guo, J. Jiang, J. Chen, J. Zhang, F. Meng and Z. Zhong, J. Controlled Release, 2022, 347, 68–77 CrossRef CAS PubMed.
- H. Qin, R. Zhao, Y. Qin, J. Zhu, L. Chen, C. Di, X. Han, K. Cheng, Y. Zhang, Y. Zhao, J. Shi, G. J. Anderson, Y. Zhao and G. Nie, Adv. Mater., 2021, 33, 2006007 Search PubMed.
- Y. Tang, X. Wang, J. Li, Y. Nie, G. Liao, Y. Yu and C. Li, ACS Nano, 2019, 13, 13015–13026 Search PubMed.
- Y. Chu, L. Qian, Y. Ke, X. Feng, X. Chen, F. Liu, L. Yu, L. Zhang, Y. Tao and R. Xu, J. Nanobiotechnol., 2022, 20, 190 Search PubMed.
- D. Zhu, Z. Liu, Y. Li, Q. Huang, L. Xia and K. Li, Biomaterials, 2021, 274, 120894 CrossRef CAS.
- K. Luo, Y. Lian, M. Zhang, H. Yu, G. Wang and J. Li, Chem. Eng. J., 2021, 412, 128659 CrossRef CAS.
- V. Sheth, L. Wang, R. Bhattacharya, P. Mukherjee and S. Wilhelm, Adv. Funct. Mater., 2021, 31, 2007363 Search PubMed.
- M. Izci, C. Maksoudian, B. B. Manshian and S. J. Soenen, Chem. Rev., 2021, 121, 1746–1803 CrossRef CAS PubMed.
- F. Mpekris, C. Voutouri, J. W. Baish, D. G. Duda, L. L. Munn, T. Stylianopoulos and R. K. Jain, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 3728–3737 CrossRef CAS.
- Y. Huang, T. Stylianopoulos, D. G. Duda, D. Fukumura and R. K. Jain, Cancer Res., 2013, 73, 7144–7146 Search PubMed.
- Y. Sun, W. Chen, R. J. Torphy, S. Yao, G. Zhu, R. Lin, R. Lugano, E. N. Miller, Y. Fujiwara, L. Bian, L. Zheng, S. Anand, F. Gao, W. Zhang, S. E. Ferrara, A. E. Goodspeed, A. Dimberg, X. J. Wang, B. H. Edil, C. C. Barnett, R. D. Schulick, L. Chen and Y. Zhu, Sci. Transl. Med., 2021, 13, eabc8922 Search PubMed.
- N. Huang, Y. Liu, Y. Fang, S. Zheng, J. Wu, M. Wang, W. Zhong, M. Shi, M. Xing and W. Liao, ACS Nano, 2020, 14, 7940–7958 Search PubMed.
- Y. Yang, J. Tang, M. Zhang, Z. Gu, H. Song, Y. Yang and C. Yu, Nano Lett., 2019, 19, 7750–7759 Search PubMed.
- R. Chen, L. Huang and K. Hu, Acta Pharm. Sin. B, 2020, 10, 2140–2155 CrossRef CAS PubMed.
- J. Zhao, H. Wang, C. H. Hsiao, D. S. Chow, E. J. Koay, Y. Kang, X. Wen, Q. Huang, Y. Ma, J. A. Bankson, S. E. Ullrich, W. Overwijk, A. Maitra, D. Piwnica-Worms, J. B. Fleming and C. Li, Biomaterials, 2018, 159, 215–228 CrossRef CAS PubMed.
- L. Zhao, B. Niu, J. Fang, Y. Pang, S. Li, C. Xie, L. Sun, X. Zhang, Z. Guo, Q. Lin and H. Chen, J. Nucl. Med., 2022, 63, 862–868 CrossRef CAS.
- K. Hu, J. Li, L. Wang, Y. Huang, L. Li, S. Ye, Y. Han, S. Huang, H. Wu, J. Su and G. Tang, Acta Pharm. Sin. B, 2022, 12, 867–875 CrossRef CAS PubMed.
- G. Sharbeen, J. A. McCarroll, A. Akerman, C. Kopecky, J. Youkhana, J. Kokkinos, J. Holst, C. Boyer, M. Erkan, D. Goldstein, P. Timpson, T. R. Cox, B. A. Pereira, J. L. Chitty, S. K. Fey, A. K. Najumudeen, A. D. Campbell, O. J. Sansom, R. M. C. Ignacio, S. Naim, J. Liu, N. Russia, J. Lee, A. Chou, A. Johns, A. J. Gill, E. Gonzales-Aloy, V. Gebski, Y. F. Guan, M. Pajic, N. Turner, M. V. Apte, T. P. Davis, J. P. Morton, K. S. Haghighi, J. Kasparian, B. J. McLean, Y. F. Setargew and P. A. Phillips, Cancer Res., 2021, 81, 3461–3479 CrossRef CAS.
- F. Mpekris, M. Panagi, C. Voutouri, J. D. Martin, R. Samuel, S. Takahashi, N. Gotohda, T. Suzuki, P. Papageorgis, P. Demetriou, C. Pierides, L. Koumas, P. Costeas, M. Kojima, G. Ishii, A. Constantinidou, K. Kataoka, H. Cabral and T. Stylianopoulos, Adv. Sci., 2021, 8, 2001917 CrossRef CAS PubMed.
- X. Liu, N. Ye, S. Liu, J. Guan, Q. Deng, Z. Zhang, C. Xiao, Z. Y. Ding, B. X. Zhang, X. P. Chen, Z. Li and X. Yang, Adv. Sci., 2021, 8, 2100233 CrossRef CAS PubMed.
- A. M. Buckley, N. Lynam-Lennon, H. O'Neill and J. O'Sullivan, Nat. Rev. Gastroenterol. Hepatol., 2020, 17, 298–313 CrossRef CAS.
- M. P. Krafft, Curr. Opin. Pharmacol., 2020, 53, 117–125 CrossRef CAS.
- Z. Wang, X. Gong, J. Li, H. Wang, X. Xu, Y. Li, X. Sha and Z. Zhang, ACS Nano, 2021, 15, 5405–5419 CrossRef CAS PubMed.
- C. Zhang, L. Yan, Z. Gu and Y. Zhao, Chem. Sci., 2019, 10, 6932–6943 RSC.
- Z. Zhang, J. Yang, Q. Min, C. Ling, D. Maiti, J. Xu, L. Qin and K. Yang, Small, 2019, 15, 1803703 CrossRef.
- W. Sang, L. Xie, G. Wang, J. Li, Z. Zhang, B. Li, S. Guo, C. X. Deng and Y. Dai, Adv. Sci., 2021, 8, 2003338 CrossRef CAS.
- X. Dai, J. Ruan, Y. Guo, Z. Sun, J. Liu, X. Bao, H. Zhang, Q. Li, C. Ye and X. Wang, Chem. Eng. J., 2021, 422, 130109 CrossRef CAS.
- X. Song, J. Xu, C. Liang, Y. Chao, Q. Jin, C. Wang, M. Chen and Z. Liu, Nano Lett., 2018, 18, 6360–6368 CrossRef CAS.
- Q. Chen, J. Chen, Z. Yang, J. Xu, L. Xu, C. Liang, X. Han and Z. Liu, Adv. Mater., 2019, 31, 1802228 CrossRef PubMed.
- L. Tian, Y. Wang, L. Sun, J. Xu, Y. Chao, K. Yang, S. Wang and Z. Liu, Matter, 2019, 1, 1061–1076 CrossRef.
- Y. Duo, Q. Liu, D. Zhu, B. Zhang, G. Luo, F. B. Wang, J. H. Chen and Y. Cao, Chem. Eng. J., 2022, 429, 132328 CrossRef CAS.
- Z. Yang, D. Gao, X. Guo, L. Jin, J. Zheng, Y. Wang, S. Chen, X. Zheng, L. Zeng, M. Guo, X. Zhang and Z. Tian, ACS Nano, 2020, 14, 17442–17457 CrossRef CAS PubMed.
- M. Shi, J. Zhang, Y. Wang, C. Peng, H. Hu, M. Qiao, X. Zhao and D. Chen, Biomaterials, 2022, 283, 121448 CrossRef CAS.
- N. Hasan, J. Lee, D. Kwak, H. Kim, A. Saparbayeva, H. J. Ahn, I. S. Yoon, M. S. Kim, Y. Jung and J. W. Yoo, Carbohydr. Polym., 2021, 270, 118387 CrossRef CAS PubMed.
- T. Kim, J. Suh, J. Kim and W. J. Kim, Adv. Sci., 2022, 9, 2101935 CrossRef CAS PubMed.
- L. Luo, Y. Qi, H. Zhong, S. Jiang, H. Zhang, H. Cai, Y. Wu, Z. Gu, Q. Gong and K. Luo, Acta Pharm. Sin. B, 2022, 12, 424–436 CrossRef CAS PubMed.
- J. Huang, C. Zhuang, J. Chen, X. Chen, X. Li, T. Zhang, B. Wang, Q. Feng, X. Zheng, M. Gong, Q. Gong, K. Xiao, K. Luo and W. Li, Adv. Mater., 2022, 34, 2201516 CrossRef CAS PubMed.
- J. Tu, K. Tu, H. Xu, L. Wang, X. Yuan, X. Qin, L. Kong, Q. Chu and Z. Zhang, Eur. J. Pharm. Biopharm., 2020, 150, 96–107 CrossRef CAS PubMed.
- D. Candas-Green, B. Xie, J. Huang, M. Fan, A. Wang, C. Menaa, Y. Zhang, L. Zhang, D. Jing, S. Azghadi, W. Zhou, L. Liu, N. Jiang, T. Li, T. Gao, C. Sweeney, R. Shen, T. Y. Lin, C. X. Pan, O. M. Ozpiskin, G. Woloschak, D. J. Grdina, A. T. Vaughan, J. M. Wang, S. Xia, A. M. Monjazeb, W. J. Murphy, L. Q. Sun, H. W. Chen, K. S. Lam, R. R. Weichselbaum and J. J. Li, Nat. Commun., 2020, 11, 4591 CrossRef CAS.
- C. M. Jackson, J. Choi and M. Lim, Nat. Immunol., 2019, 20, 1100–1109 CrossRef CAS PubMed.
- L. Feng, L. Yang, L. Li, J. Xiao, N. Bie, C. Xu, J. Zhou, H. Liu, L. Gan and Y. Wu, Nano Res., 2022, 15, 593–602 CrossRef CAS.
- L. You, W. Wu, X. Wang, L. Fang, V. Adam, E. Nepovimova, Q. Wu and K. Kuca, Med. Res. Rev., 2021, 41, 1622–1643 CrossRef CAS PubMed.
- K. Roehle, L. Qiang, K. S. Ventre, D. Heid, L. R. Ali, P. Lenehan, M. Heckler, S. J. Crowley, C. T. Stump, G. Ro, A. Godicelj, A. M. Bhuiyan, A. Yang, M. Quiles Del Rey, T. Biary, A. M. Luoma, P. T. Bruck, J. F. Tegethoff, S. L. Nopper, J. Li, K. T. Byrne, M. Pelletier, K. W. Wucherpfennig, B. Z. Stanger, J. J. Akin, J. D. Mancias, J. Agudo, M. Dougan and S. K. Dougan, Sci. Transl. Med., 2021, 13, eabf5058 CrossRef CAS PubMed.
- J. Yu, M. D. Green, S. Li, Y. Sun, S. N. Journey, J. E. Choi, S. M. Rizvi, A. Qin, J. J. Waninger, X. Lang, Z. Chopra, I. El Naqa, J. Zhou, Y. Bian, L. Jiang, A. Tezel, J. Skvarce, R. K. Achar, M. Sitto, B. S. Rosen, F. Su, S. P. Narayanan, X. Cao, S. Wei, W. Szeliga, L. Vatan, C. Mayo, M. A. Morgan, C. A. Schonewolf, K. Cuneo, I. Kryczek, V. T. Ma, C. D. Lao, T. S. Lawrence, N. Ramnath, F. Wen, A. M. Chinnaiyan, M. Cieslik, A. Alva and W. Zou, Nat. Med., 2021, 27, 152–164 CrossRef CAS PubMed.
- M. De Martino, O. Padilla, C. Daviaud, C.-C. Wu, R. D. Gartrell and C. Vanpouille-Box, Front. Oncol., 2021, 11, 671044 CrossRef PubMed.
- C. Groth, X. Hu, R. Weber, V. Fleming, P. Altevogt, J. Utikal and V. Umansky, Brit. J. Cancer, 2019, 120, 16–25 CrossRef CAS PubMed.
- M. Oshi, Y. Tokumaru, M. Asaoka, L. Yan, V. Satyananda, R. Matsuyama, N. Matsuhashi, M. Futamura, T. Ishikawa, K. Yoshida, I. Endo and K. Takabe, Sci. Rep., 2020, 10, 16554 CrossRef CAS PubMed.
- H. Grégoire, L. Roncali, A. Rousseau, M. Chérel, Y. Delneste, P. Jeannin, F. Hindré and E. Garcion, Front. Pharmacol., 2020, 11, 368 CrossRef PubMed.
- Z. Li, Y. Ding, J. Liu, J. Wang, F. Mo, Y. Wang, T. J. Chen-Mayfield, P. M. Sondel, S. Hong and Q. Hu, Nat. Commun., 2022, 13, 1845 CrossRef CAS PubMed.
- X. Dai, L. Ren, M. Liu, H. Cai, H. Zhang, Q. Gong, Z. Gu and K. Luo, Nano Today, 2021, 39, 101163 CrossRef CAS.
- N. T. Trac and E. J. Chung, Bioact. Mater., 2020, 5, 92–101 Search PubMed.
- C. Ngambenjawong, H. H. Gustafson and S. H. Pun, Adv. Drug Delivery Rev., 2017, 114, 206–221 Search PubMed.
- L. Tian, X. Yi, Z. Dong, J. Xu, C. Liang, Y. Chao, Y. Wang, K. Yang and Z. Liu, ACS Nano, 2018, 12, 11541–11551 CrossRef CAS PubMed.
- W. Huang, L. He, J. Ouyang, Q. Chen, C. Liu, W. Tao and T. Chen, Matter, 2020, 3, 1725–1753 CrossRef.
- F. Castro, M. L. Pinto, C. L. Pereira, K. Serre, M. A. Barbosa, K. Vermaelen, F. Gärtner, R. M. Gonçalves, O. De Wever and M. J. Oliveira, Biomaterials, 2020, 257, 120218 CrossRef CAS PubMed.
- P. Zhang, J. Miska, C. Lee-Chang, A. Rashidi, W. K. Panek, S. An, M. Zannikou, A. Lopez-Rosas, Y. Han, T. Xiao, K. C. Pituch, D. Kanojia, I. V. Balyasnikova and M. S. Lesniak, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 23714–23723 CrossRef CAS PubMed.
- Y. D. Zhao, M. Muhetaerjiang, H. W. An, X. Fang, Y. Zhao and H. Wang, Biomaterials, 2021, 268, 120552 CrossRef CAS PubMed.
- M. A. Sallam, C. Wyatt Shields Iv, S. Prakash, J. Kim, D. C. Pan and S. Mitragotri, J. Controlled Release, 2021, 335, 333–344 CrossRef CAS PubMed.
- S. Theivendran, Z. Gu, J. Tang, Y. Yang, H. Song, Y. Yang, M. Zhang, D. Cheng and C. Yu, ACS Nano, 2022, 16, 10943–10957 CrossRef CAS PubMed.
- W. Zhang, Y. Shi, H. Li, M. Yu, J. Zhao, H. Chen and M. Kong, Carbohydr. Polym., 2022, 288, 119418 CrossRef CAS PubMed.
- N. Qiu, G. Wang, J. Wang, Q. Zhou, M. Guo, Y. Wang, X. Hu, H. Zhou, R. Bai, M. You, Z. Zhang, C. Chen, Y. Liu and Y. Shen, Adv. Mater., 2021, 33, 2100137 CrossRef CAS PubMed.
- M. Cao, H. Yan, X. Han, L. Weng, Q. Wei, X. Sun, W. Lu, Q. Wei, J. Ye, X. Cai, C. Hu, X. Yin and P. Cao, J. Immunother. Cancer, 2019, 7, 326 CrossRef PubMed.
- K. Ni, T. Luo, A. Culbert, M. Kaufmann, X. Jiang and W. Lin, J. Am. Chem. Soc., 2020, 142, 12579–12584 CrossRef CAS PubMed.
- D. W. Ho, Y. M. Tsui, L. K. Chan, K. M. Sze, X. Zhang, J. W. Cheu, Y. T. Chiu, J. M. Lee, A. C. Chan, E. T. Cheung, D. T. Yau, N. H. Chia, I. L. Lo, P. C. Sham, T. T. Cheung, C. C. Wong and I. O. Ng, Nat. Commun., 2021, 12, 3684 CrossRef CAS PubMed.
- X. Li, P. Ramadori, D. Pfister, M. Seehawer, L. Zender and M. Heikenwalder, Nat. Rev. Cancer, 2021, 21, 541–557 CrossRef CAS PubMed.
- M. Mondini, P. L. Loyher, P. Hamon, M. Gerbé de Thoré, M. Laviron, K. Berthelot, C. Clémenson, B. L. Salomon, C. Combadière, E. Deutsch and A. Boissonnas, Cancer Immunol. Res., 2019, 7, 376–387 CrossRef CAS PubMed.
- Y. Lan, D. Zhang, C. Xu, K. W. Hance, B. Marelli, J. Qi, H. Yu, G. Qin, A. Sircar, V. M. Hernández, M. H. Jenkins, R. E. Fontana, A. Deshpande, G. Locke, H. Sabzevari, L. Radvanyi and K. M. Lo, Sci. Transl. Med., 2018, 10, eaan5488 CrossRef PubMed.
- C. Mao, S. Yeh, J. Fu, M. Porosnicu, A. Thomas, G. L. Kucera, K. I. Votanopoulos, S. Tian and X. Ming, Sci. Transl. Med., 2022, 14, eabh1261 CrossRef CAS PubMed.
- B. G. Kim, E. Malek, S. H. Choi, J. J. Ignatz-Hoover and J. J. Driscoll, J. Hematol. Oncol., 2021, 14, 55 CrossRef CAS PubMed.
- M. Lin, Z. Yang, Y. Yang, Y. Peng, J. Li, Y. Du, Q. Sun, D. Gao, Q. Yuan, Y. Zhou, X. Chen and X. Qi, Nano Today, 2022, 42, 101359 CrossRef CAS.
- Y. Zhu, B. L. Knolhoff, M. A. Meyer, T. M. Nywening, B. L. West, J. Luo, A. Wang-Gillam, S. P. Goedegebuure, D. C. Linehan and D. G. DeNardo, Cancer Res., 2014, 74, 5057–5069 CrossRef CAS PubMed.
- A. Kalbasi, C. Komar, G. M. Tooker, M. Liu, J. W. Lee, W. L. Gladney, E. Ben-Josef and G. L. Beatty, Clin. Cancer Res., 2017, 23, 137–148 CrossRef CAS PubMed.
- X. Cao, J. Chen, B. Li, J. Dang, W. Zhang, X. Zhong, C. Wang, M. Raoof, Z. Sun, J. Yu, M. G. Fakih and M. Feng, Sci. Adv., 2022, 8, eabl9171 CrossRef CAS PubMed.
- M. S. Alghamri, K. Banerjee, A. A. Mujeeb, A. Mauser, A. Taher, R. Thalla, B. L. McClellan, M. L. Varela, S. M. Stamatovic, G. Martinez-Revollar, A. V. Andjelkovic, J. V. Gregory, P. Kadiyala, A. Calinescu, J. A. Jiménez, A. A. Apfelbaum, E. R. Lawlor, S. Carney, A. Comba, S. M. Faisal, M. Barissi, M. B. Edwards, H. Appelman, Y. Sun, J. Gan, R. Ackermann, A. Schwendeman, M. Candolfi, M. R. Olin, J. Lahann, P. R. Lowenstein and M. G. Castro, ACS Nano, 2022, 16, 8729–8750 CrossRef CAS PubMed.
- D. De Ruysscher, G. Niedermann, N. G. Burnet, S. Siva, A. W. M. Lee and F. Hegi-Johnson, Nat. Rev. Dis. Primers, 2019, 5, 13 CrossRef.
- J. Constanzo, J. Faget, C. Ursino, C. Badie and J. P. Pouget, Front. Immunol., 2021, 12, 680503 CrossRef CAS.
- A. Cohen, K. Ioannidis, A. Ehrlich, S. Regenbaum, M. Cohen, M. Ayyash, S. S. Tikva and Y. Nahmias, Sci. Transl. Med., 2021, 13, eabd6299 CrossRef CAS PubMed.
- D. B. Johnson, K. L. Reynolds, R. J. Sullivan, J. M. Balko, J. R. Patrinely, L. C. Cappelli, J. Naidoo and J. J. Moslehi, Lancet Oncol., 2020, 21, 398–404 CrossRef.
- L. B. Kennedy and A. K. S. Salama, CA-Cancer J. Clin., 2020, 70, 86–104 CrossRef.
- J. Luo, J. A. Beattie, P. Fuentes, H. Rizvi, J. V. Egger, J. A. Kern, D. Y. M. Leung, M. E. Lacouture, M. G. Kris, M. Gambarin, B. D. Santomasso, D. M. Faleck and M. D. Hellmann, J. Thorac. Oncol., 2021, 16, 1759–1764 CrossRef CAS PubMed.
- P. D. Brown, V. Gondi, S. Pugh, W. A. Tome, J. S. Wefel, T. S. Armstrong, J. A. Bovi, C. Robinson, A. Konski, D. Khuntia, D. Grosshans, T. L. S. Benzinger, D. Bruner, M. R. Gilbert, D. Roberge, V. Kundapur, K. Devisetty, S. Shah, K. Usuki, B. M. Anderson, B. Stea, H. Yoon, J. Li, N. N. Laack, T. J. Kruser, S. J. Chmura, W. Shi, S. Deshmukh, M. P. Mehta and L. A. Kachnic, J. Clin. Oncol., 2020, 38, 1019–1029 CrossRef PubMed.
- M. Karami, S. Asri-Rezaei, B. Dormanesh and A. Nazarizadeh, Int. J. Radiat. Biol., 2018, 94, 17–27 Search PubMed.
- J. B. Maxhimer, D. R. Soto-Pantoja, L. A. Ridnour, H. B. Shih, W. G. Degraff, M. Tsokos, D. A. Wink, J. S. Isenberg and D. D. Roberts, Sci. Transl. Med., 2009, 1, 3ra7 Search PubMed.
- S. Gerassy-Vainberg, A. Blatt, Y. Danin-Poleg, K. Gershovich, E. Sabo, A. Nevelsky, S. Daniel, A. Dahan, O. Ziv, R. Dheer, M. T. Abreu, O. Koren, Y. Kashi and Y. Chowers, Gut, 2018, 67, 97–107 CrossRef CAS.
- W. Lu, Y. Xie, B. Huang, T. Ma, H. Wang, B. Deng, S. Zou, W. Wang, Q. Tang, Z. Yang, X. Li, L. Wang and L. Fang, Sci. Transl. Med., 2021, 13, eabc2344 CrossRef CAS.
- Y. Zhao, Y. Q. Xie, S. Van Herck, S. Nassiri, M. Gao, Y. Guo and L. Tang, Sci. Adv., 2021, 7, eabg7291 CrossRef CAS.
- W. J. Smith, G. Wang, H. Gaikwad, V. P. Vu, E. Groman, D. W. A. Bourne and D. Simberg, ACS Nano, 2018, 12, 12523–12532 CrossRef CAS PubMed.
- R. Yudistiro, H. Hanaoka, N. Katsumata, A. Yamaguchi and Y. Tsushima, Mol. Pharm., 2018, 15, 2165–2173 CrossRef CAS PubMed.
- S. M. Cheal, M. Patel, G. Yang, D. Veach, H. Xu, H. F. Guo, P. B. Zanzonico, D. B. Axworthy, N. V. Cheung, O. Ouerfelli and S. M. Larson, Bioconjugate Chem., 2020, 31, 501–506 CrossRef CAS.
- S. M. Sarrett, O. Keinänen, E. J. Dayts, G. Dewaele-Le Roi, C. Rodriguez, K. E. Carnazza and B. M. Zeglis, Nat. Protoc., 2021, 16, 3348–3381 CrossRef CAS.
- G. Parisi, J. D. Saco, F. B. Salazar, J. Tsoi, P. Krystofinski, C. Puig-Saus, R. Zhang, J. Zhou, G. C. Cheung-Lau, A. J. Garcia, C. S. Grasso, R. Tavaré, S. Hu-Lieskovan, S. Mackay, J. Zalevsky, C. Bernatchez, A. Diab, A. M. Wu, B. Comin-Anduix, D. Charych and A. Ribas, Nat. Commun., 2020, 11, 660 CrossRef CAS PubMed.
- C. Stavnsbjerg, E. Christensen, R. Münter, J. R. Henriksen, M. Fach, L. Parhamifar, C. Christensen, A. Kjaer, A. E. Hansen and T. L. Andresen, J. Controlled Release, 2021, 342, 337–344 CrossRef PubMed.
- T. Kordbacheh, J. Honeychurch, F. Blackhall, C. Faivre-Finn and T. Illidge, Ann. Oncol., 2018, 29, 301–310 CrossRef CAS PubMed.
- B. E. Lippitz and R. A. Harris, Radiother. Oncol., 2019, 140, 116–124 CrossRef CAS.
- C. Pérez-Medina, A. J. P. Teunissen, E. Kluza, W. J. M. Mulder and R. van der Meel, Adv. Drug Delivery Rev., 2020, 154–155, 123–141 CrossRef.
- A. A. Gabizon, R. T. M. de Rosales and N. M. La-Beck, Adv. Drug Delivery Rev., 2020, 158, 140–157 CrossRef CAS PubMed.
- S. N. Bhatia, X. Chen, M. A. Dobrovolskaia and T. Lammers, Nat. Rev. Cancer, 2022, 22, 550–556 CrossRef CAS.
- L. Yin, J. Xue, R. Li, L. Zhou, L. Deng, L. Chen, Y. Zhang, Y. Li, X. Zhang, W. Xiu, R. Tong, Y. Gong, M. Huang, Y. Xu, J. Zhu, M. Yu, M. Li, J. Lan, J. Wang, X. Mo, Y. Wei, G. Niedermann and Y. Lu, Int. J. Radiat. Oncol. Biol. Phys., 2020, 108, 212–224 CrossRef.
- J. Lin, M. Yin, X. Liu, F. Meng and L. Luo, Macromol. Rapid Commun., 2022, 43, 2200194 CrossRef CAS.
- B. Cabanillas, N. Novak and C. A. Akdis, Allergy, 2022, 77, 1658–1660 CrossRef CAS PubMed.
- G. T. Kozma, T. Shimizu, T. Ishida and J. Szebeni, Adv. Drug Delivery Rev., 2020, 154–155, 163–175 CrossRef CAS PubMed.
- J. Zhao, G. Hu, Y. Huang, Y. Huang, X. Wei and J. Shi, Chin. Chem. Lett., 2021, 32, 1331–1340 CrossRef CAS.
- D. Zhai, J. Huang, Y. Hu, C. Wan, Y. Sun, J. Meng, H. Zi, L. Lu, Q. He, Y. Hu, H. Jin and K. Yang, Int. J. Radiat. Oncol. Biol. Phys., 2022, 114, 502–515 CrossRef.
- Y. Wang, Q. Zhao, B. Zhao, Y. Zheng, Q. Zhuang, N. Liao, P. Wang, Z. Cai, D. Zhang, Y. Zeng and X. Liu, Adv. Sci., 2022, 9, 2105631 CrossRef CAS.
- J. Zhou, A. V. Kroll, M. Holay, R. H. Fang and L. Zhang, Adv. Mater., 2020, 32, 1901255 CrossRef CAS PubMed.
- D. C. St Germain and W. McCaskill-Stevens, Cancer, 2021, 127, 1630–1637 CrossRef PubMed.
- C. Tang, S. I. Sherman, M. Price, J. Weng, S. E. Davis, D. S. Hong, J. C. Yao, A. Buzdar, G. Wilding and J. J. Lee, Clin. Cancer Res., 2017, 23, 1414–1421 CrossRef PubMed.
- M. Souri, M. Soltani, F. Moradi Kashkooli, M. Kiani Shahvandi, M. Chiani, F. S. Shariati, M. R. Mehrabi and L. L. Munn, Mater. Today Bio., 2022, 13, 100208 CrossRef CAS PubMed.
- Y. Min, J. M. Caster, M. J. Eblan and A. Z. Wang, Chem. Rev., 2015, 115, 11147–11190 CrossRef CAS.
- G. Sriram, L. E. Milling, J. K. Chen, Y. W. Kong, B. A. Joughin, W. Abraham, S. Swartwout, E. D. Handly, D. J. Irvine and M. B. Yaffe, Sci. Signal., 2021, 14, eabc4764 CrossRef CAS.
- B. Olson, Y. Li, Y. Lin, E. T. Liu and A. Patnaik, Cancer Discovery, 2018, 8, 1358–1365 CrossRef CAS.
- S. V. Paffenholz, C. Salvagno, Y. J. Ho, M. Limjoco, T. Baslan, S. Tian, A. Kulick, E. de Stanchina, J. E. Wilkinson, F. M. Barriga, D. Zamarin, J. R. Cubillos-Ruiz, J. Leibold and S. W. Lowe, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2117754119 CrossRef CAS.
- C. Verry, S. Dufort, B. Lemasson, S. Grand, J. Pietras, I. Troprès, Y. Crémillieux, F. Lux, S. Mériaux, B. Larrat, J. Balosso, G. Le Duc, E. L. Barbier and O. Tillement, Sci. Adv., 2020, 6, eaay5279 CrossRef CAS PubMed.
- T. I. Janjua, Y. Cao, C. Yu and A. Popat, Nat. Rev. Mater., 2021, 6, 1072–1074 CrossRef CAS.
- C. Liu, X. Liu, X. Xiang, X. Pang, S. Chen, Y. Zhang, E. Ren, L. Zhang, X. Liu, P. Lv, X. Wang, W. Luo, N. Xia, X. Chen and G. Liu, Nat. Nanotechnol., 2022, 17, 531–540 CrossRef CAS PubMed.
- Z. Chen, H. Pan, Y. Luo, T. Yin, B. Zhang, J. Liao, M. Wang, X. Tang, G. Huang and G. Deng, Small, 2021, 17, 2007494 CrossRef CAS.
- B. H. Santich, S. M. Cheal, M. Ahmed, M. R. McDevitt, O. Ouerfelli, G. Yang, D. R. Veach, E. K. Fung, M. Patel, D. Burnes Vargas, A. A. Malik, H. F. Guo, P. B. Zanzonico, S. Monette, A. O. Michel, C. M. Rudin, S. M. Larson and N. K. Cheung, Clin. Cancer Res., 2021, 27, 532–541 CrossRef CAS.
- X. Wen, X. Zeng, X. Cheng, X. Zeng, J. Liu, Y. Zhang, Y. Li, H. Chen, J. Huang, Z. Guo, X. Chen and X. Zhang, Mol. Pharm., 2022, 19, 3612–3622 CrossRef CAS.
- U. Eberlein, M. Cremonesi and M. Lassmann, J. Nucl. Med., 2017, 58, 97s–103s CrossRef CAS.
- S. M. Qaim, B. Scholten and B. Neumaier, J. Radioanal. Nucl. Chem., 2018, 318, 1493–1509 CrossRef CAS.
- F. Man, P. J. Gawne and R. T. M. de Rosales, Adv. Drug Delivery Rev., 2019, 143, 134–160 CrossRef CAS.
- H. Deng, W. Yang, Z. Zhou, R. Tian, L. Lin, Y. Ma, J. Song and X. Chen, Nat. Commun., 2020, 11, 4951 CrossRef CAS PubMed.
- L. M. Coussens and Z. Werb, Nature, 2002, 420, 860–867 Search PubMed.
- M. McLaughlin, E. C. Patin, M. Pedersen, A. Wilkins, M. T. Dillon, A. A. Melcher and K. J. Harrington, Nat. Rev. Cancer, 2020, 20, 203–217 CrossRef CAS PubMed.
- L. Shi, J. Wang, N. Ding, Y. Zhang, Y. Zhu, S. Dong, X. Wang, C. Peng, C. Zhou, L. Zhou, X. Li, H. Shi, W. Wu, X. Long, C. Wu and W. Liao, Nat. Commun., 2019, 10, 5421 CrossRef.
- M. M. Wattenberg and G. L. Beatty, Semin. Cancer Biol., 2020, 65, 38–50 CrossRef CAS.
- R. Weissleder and M. J. Pittet, Nat. Biomed. Eng., 2020, 4, 489–498 CrossRef CAS PubMed.
- L. M. Coussens, L. Zitvogel and A. K. Palucka, Science, 2013, 339, 286–291 CrossRef CAS.
- R. J. Pickering and C. E. Bryant, Science, 2020, 369, 1564–1565 CrossRef CAS.
- X. Wang, P. Hua, C. He and M. Chen, Acta Pharm. Sin. B, 2022, 12, 3567–3593 CrossRef CAS.
- Y. Liu, W. Zhen, Y. Wang, S. Song and H. Zhang, J. Am. Chem. Soc., 2020, 142, 21751–21757 CrossRef CAS.
- B. Ma, A. Wells, L. Wei and J. Zheng, Semin. Cancer Biol., 2021, 71, 2–9 CrossRef CAS PubMed.
- M. E. Giuliani, C. G. Robinson, J. K. Salama and M. E. Daly, Int. J. Radiat. Oncol. Biol. Phys., 2020, 106, 455–459 CrossRef.
- M. Guckenberger, Y. Lievens, A. B. Bouma, L. Collette, A. Dekker, N. M. deSouza, A. C. Dingemans, B. Fournier, C. Hurkmans, F. E. Lecouvet, I. Meattini, A. Méndez Romero, U. Ricardi, N. S. Russell, D. H. Schanne, M. Scorsetti, B. Tombal, D. Verellen, C. Verfaillie and P. Ost, Lancet Oncol., 2020, 21, 18–28 CrossRef.
- W. Zhang, F. Wang, C. Hu, Y. Zhou, H. Gao and J. Hu, Acta Pharm. Sin. B, 2020, 10, 2037–2053 CrossRef CAS.
- X. Kong, R. Cheng, J. Wang, Y. Fang and K. C. Hwang, Nano Today, 2021, 36, 101004 CrossRef CAS.
- Y. Liu, W. N. Crowe, L. Wang, Y. Lu, W. J. Petty, A. A. Habib and D. Zhao, Nat. Commun., 2019, 10, 5108 CrossRef CAS.
- Y. Li, X. Zhao, X. Liu, K. Cheng, X. Han, Y. Zhang, H. Min, G. Liu, J. Xu, J. Shi, H. Qin, H. Fan, L. Ren and G. Nie, Adv. Mater., 2020, 32, 1906799 CrossRef CAS.
- M. Li, D. Liu, D. Lee, S. Kapoor, K. N. Gibson-Corley, T. P. Quinn, E. A. Sagastume, S. L. Mott, S. A. Walsh, M. R. Acevedo, F. L. Johnson and M. K. Schultz, Mol. Pharm., 2019, 16, 3904–3915 CrossRef CAS.
- M. Singhal, N. Gengenbacher, A. A. Abdul Pari, M. Kamiyama, L. Hai, B. J. Kuhn, D. M. Kallenberg, S. R. Kulkarni, C. Camilli, S. F. Preuß, B. Leuchs, C. Mogler, E. Espinet, E. Besemfelder, D. Heide, M. Heikenwalder, M. R. Sprick, A. Trumpp, J. Krijgsveld, M. Schlesner, J. Hu, S. E. Moss, J. Greenwood and H. G. Augustin, Sci. Transl. Med., 2021, 13, eabe6805 CrossRef.
- G. Bergers and S. M. Fendt, Nat. Rev. Cancer, 2021, 21, 162–180 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |