News and Views
Running fuel cells on biogas — a renewable fuel
Highlights
Centre for Green Chemistry, Monash University
From plant to plastic,
Renewables are fantastic!
Running fuel cells on biogas — a renewable fuel
Biogas represents a renewable but largely under-exploited energy reserve often ignored because of the variable composition and often low level of methane that prevents its use in conventional power systems. As part of our continuing series of front-section articles on renewable waste energy, John Staniforth and Mark Ormerod from the Birchall Centre for Inorganic Chemistry and Materials Science at Keele University, UK, describe how biogas can be used directly in a solid oxide fuel cell even at remarkably low levels of methane thus offering a valuable use for poor-quality biogas that is currently wasted.
| Professor Mark Ormerod is an EPSRC Advanced Research Fellow and is Professor of Catalysis and Materials Chemistry. He and Dr John Staniforth are based in the Birchall Centre for Inorganic Chemistry and Materials Science, at Keele University, UK. |
Introduction to biogas
Biogas is a complex and variable mixture of methane, carbon dioxide and other gases.1 It is cheap and readily available and should be considered as a very under-exploited energy reserve. It is currently used for heating and cooking purposes in Third World countries such as India and China.2 One of the principal limitations of biogas in certain applications is its variability of composition, not only in different locations but also over time, which presents major difficulties in its use.3 As the proportion of carbon dioxide in the biogas increases the fuel becomes progressively more difficult to ignite, and eventually the proportion of CO2 becomes such that ignition can no longer be maintained; this occurs when the ratio of CO2 to methane is about 3∶1.4 Because of this situation, large quantities of biogas are presently vented to the atmosphere, making a significant contribution to greenhouse gas emissions, whilst at the same time wasting a potentially clean, renewable energy resource.Solid oxide fuel cells (SOFCs)
Fuel cells are currently attracting tremendous interest because of their huge potential in stationary, portable and transport applications,5-9 in terms of sustainability of our energy use. They also offer environmental advantages, combining significantly higher efficiency with very much lower emissions of SOx, NOx and residual hydrocarbons, and significantly reduced CO2 emissions, compared to conventional power generation. Solid oxide fuel cells (SOFCs) (which use a hard ceramic material instead of a liquid electrolyte, and operate at a very high temperature) offer potential advantages in terms of efficiency, flexibility and cost over other types of fuel cells, because of their tolerance to carbon monoxide, their increased resistance to poisons and impurities in the fuel and because their high operating temperatures allow the possibility of running the fuel cell directly on natural gas or other practical hydrocarbon fuels, catalytically reforming the fuel to CO and H2 within the fuel cell.8,9 It is generally accepted that for SOFCs to be cost-effective, internal reforming of the fuel within the fuel cell is essential, since this both increases efficiency and reduces the complexity of the system.8 Although natural gas is the most common fuel for SOFCs, other hydrocarbon fuels such as propane and butane are particularly suitable for certain applications.Biogas as a fuel source for SOFCs
From the above it follows that biogas can be considered as a possible source of fuel for the solid oxide fuel cell. At CO2 levels which are too high for conventional power generation systems, SOFCs could, in theory, still extract the power available from the methane content of biogas. Thus, in principle, SOFCs offer the possibility of using even biogas that is depleted in methane, consuming methane which would otherwise be vented wastefully and harmfully to the atmosphere, thus acting as an environmental clean-up device, significantly reducing the contribution to greenhouse gas emissions (methane being a 26-fold greenhouse gas than CO2), whilst at the same time producing useful energy.Conventionally in SOFCs steam is added to the natural gas and the natural gas is catalytically converted to CO and H2via steam reforming [eqn. (1)], either externally, or preferably internally, either indirectly using a reforming catalyst or directly on the nickel anode.10
| CH4 + H2O → 3H2 + CO | (1) |
| CH4 + 1/2O2→ 2H2 + CO | (2) |
| CH4 + CO2→ 2H2 + 2CO | (3) |
SOFC test system
At Keele we have recently demonstrated that it is possible to run a solid oxide fuel cell directly on biogas, using a small tubular SOFC and test system developed in our laboratory.The SOFC test system, shown schematically in Fig. 1, is based on a small diameter, thin-walled tubular solid electrolyte reactor. The test cell inlet is linked to a gas handling system which enables evaluation over a full range of operating conditions and fuel compositions. The exhaust gas from the SOFC is monitored by gas chromatography. Hence, the test system allows the performance and durability of the fuel cell to be evaluated, whilst simultaneously directly studying the reforming catalysis and reactions of biogas in the actual working SOFC. We have previously demonstrated the value of such in situ catalytic measurements on SOFCs.14
 | ||
| Fig. 1 Schematic of a solid oxide fuel cell running on biogas. | ||
The SOFC has a two-layer anode, both layers consisting of nickel oxide and yttria-stabilised zirconia physically mixed in specified proportions in a solvent slurry. Ceria was also added to the outer layer anode. The anode was applied to the inside of the electrolyte tubes and dried in air before high temperature firing. Strontium-doped lanthanum manganite was used as the cathode material and again applied in two layers. Gas mixtures were made up to cover the full composition range of all biogas sources. The anodes were not pre-reduced but were exposed directly to the biogas feed at operating temperature.
Fig. 2 shows the power obtained from an SOFC operating at 850 °C as a function of methane content of the biogas, whilst Fig. 3 shows the corresponding exit gas composition from the SOFC under the same experimental conditions, except that the measurements were made with the cell not under load in order to prevent interference from the electrochemical cell reactions. The changes in the power output can be attributed to the gas composition at the anode and hence the availability and type of fuel.
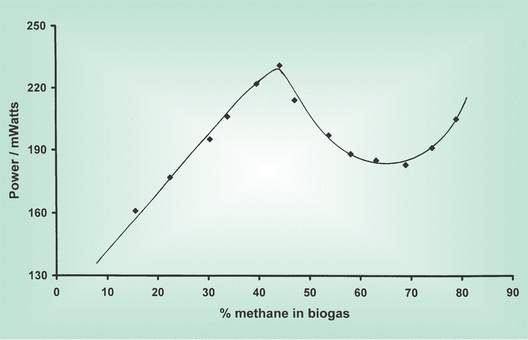 | ||
| Fig. 2 Power output from a small tubular solid oxide fuel cell running on biogas at 850 °C, as a function of methane content. | ||
 | ||
| Fig. 3 Exit gas compositions from an unloaded solid oxide fuel cell running on biogas at 850 °C, as a function of methane content. | ||
There is a steady increase in power output from the cell over the range 15–45% methane content in the biogas, with a maximum power output for biogas with a methane content of 45%. As the methane content in the biogas exceeds 45% the power output decreases, initially quite quickly and then more slowly, reaching a minimum for biogas with a methane content around 70%, with an increase in power output being observed for biogas with methane content exceeding 70%.
For biogas samples with methane contents of 15–45% the exit gas composition from the fuel cell shows that complete consumption of the methane occurs, together with the formation of H2 and CO, the amount of which parallel the increase in power output, and significant consumption of the CO2 in the biogas. Maximum formation of CO and H2 occurs at almost exactly the same fuel composition at which maximum power output is observed, close to equimolar quantities of methane and CO2. At this biogas composition essentially complete consumption of CO2 was observed. The exit gas compositions thus clearly show that internal dry reforming of the methane present in the biogas [eqn. (3)] is occurring effectively. As the methane content of the biogas exceeds 45% and the CO2 content decreases, the amount of H2 and CO formed start to decrease. This behaviour again precisely mirrors the power output over this composition range. The quantity of methane in the exit gas continues to increase. This clearly demonstrates that it is electrochemical oxidation of H2 and CO which lead to power production, and that direct electrochemical oxidation of methane does not therefore make any significant contribution to power production.
For biogas with methane content around 75% the amount of H2 in the exit gas reaches a minimum, with H2 production increasing at higher methane contents, with a parallel increase in the power output from the cell also being observed. This can be rationalised in terms of catalytic methane decomposition [eqn. (4)] becoming progressively more important at high methane levels, providing an additional route to H2 production, which is then electrochemically oxidised.
| CH4→ C(ad) + 2H2 | (4) |
| CO2 + C(ad)→ 2CO | (5) |
Conclusion
We have shown that it is possible to run a solid oxide fuel cell directly on biogas a truly renewable fuel, over a wide compositional range of methane and carbon dioxide. It is particularly worth noting that the amount of power produced within the SOFC from biogas is still very reasonable at methane contents as low as 15%, being ∼70% of the maximum power output. Thus at methane contents below which conventional heat engines would have long since stopped working (∼45%), the SOFC is able not only to function but to produce a significant amount of power from poor-quality biogas, which is presently disposed of by simply venting wastefully and detrimentally to the atmosphere. Electricity and useful energy are produced, with significant reduction in greenhouse gas emissions. Our current research is directed towards longer-term durability experiments using biogas, with a particular emphasis on biogas with a low methane content.15References
- M. Hammad, D. Badarneh and K. Tahboub, Energy Convers. Manage., 1999, 40, 1463 CrossRef CAS.
- C. Bell, S. Boulter, D. Dunlop and P. Keiller, in Methane: Fuel of the Future, 1973Andrew Singer, Bottisham, UK. Search PubMed.
- J. Huang and R. J. Crookes, Fuel, 1998, 77, 1793 CrossRef CAS.
- S. Neyeloff and W. W. Gungel, in Energy, Agriculture and Waste Management, Ann Arbor Science, 1975. Search PubMed.
- R. Kingston, Chem. Brit., June 2000, 24. Search PubMed.
- G. Hoogers and D. Thompsett, Chem. Ind., 1999, 796. Search PubMed.
- C. K. Dyer, Sci. Am., 1999, 281, 70 Search PubMed.
- D. Hart, Chem. Ind., 1998, 344. Search PubMed.
- A. C. Lloyd, Sci. Am., 1999, 281, 64 Search PubMed.
- C. M. Finnerty, N. J. Coe, R. H. Cunningham and R. M. Ormerod, Catal. Today, 1998, 46, 137 CrossRef CAS.
- C. M. Finnerty and R. M. Ormerod, J. Power Sources, 2000, 86, 390 CrossRef CAS.
- A. T. Ashcroft, A. K. Cheetham, J. S. Foord, M. L. H. Green, C. P. Grey, A. J. Murrell and P. D. F. Vernon, Nature, 1990, 344, 319 CrossRef CAS.
- F. Besenbacher, I. Chorkendorff, B. S. Clausen, B. Hammer, A. M. Molenbroek, J. K. Norskov and I. Stensgaard, Science, 1998, 279, 1913 CrossRef CAS.
- C. M. Finnerty, R. H. Cunningham, K. Kendall and R. M. Ormerod, Chem. Commun., 1998, 915. Search PubMed.
- J. Staniforth and R. M. Ormerod, in preparation.
Highlights
Duncan Macquarrie reviews the latest research in green chemistry
Mannich reactions
The Mannich reaction represents a powerful method for the formation of advanced synthons, especially when carried out in an enantioselective manner. Two papers from the group led by Karl Anker Jørgensen of Aarhus University in Denmark are of great interest in this respect. The first (Angew. Chem., Int. Ed., 2001, 40, 2992) describes the addition of nitroalkanes to iminoesters, using chiral bisoxazoline copper catalysts. This protocol allows the formation of the addition products in high yield (typically >80%) and with de and ee both above 90%. The fact that the reactions can be run at, or close to, room temperature avoids the need for difficult (and energy-intensive) cooling to the very low temperatures often required for such enantioselective methodologies. A range of nitro compounds have been used and, while the de can vary significantly, the ee remains high. | ||
| Scheme 1 | ||
The second paper describes the direct Mannich reaction of carbonyl compounds with imino esters, again utilising the bisoxazoline copper catalysts described in the first paper (Angew. Chem., Int. Ed., 2001, 40, 2995). Here again the reactions are carried out advantageously at or around ambient temperature, and the ee’s and de’s are typically good. A further important feature of the reaction, perhaps the most innovative, is that the carbonyl component can be used directly, avoiding the wasteful preparation of very sensitive silyl enol esters. This makes the procedure shorter, more robust and much less wasteful.
 | ||
| Scheme 2 | ||
LASC technique
Another contribution to synthetic methodology has been provided by the group of Shu Kobayashi in the University of Tokyo (Angew. Chem., Int. Ed., 2001, 40, 2815). This research involved the use of the so-called Lewis Acid Surfactant Combined catalyst (LASC) technique, which has already been successfully applied to other C–C bond-forming processes. In this process, colloidal particles are formed in water, inside which the desired reaction takes place. In this case, the reaction is the Mukaiyama aldol reaction of a silyl enol ether and an aldehyde, catalysed by diphenylboronic acid and 1 mol% of benzoic acid. Yields are generally high, and diastereo- selectivity was also very good in most cases. The reaction proceeds via exchange of the silyl group for the boron species, making the real reagent a boron enolate. The method described represents the first example where such species can be formed catalytically in situ, and thus may prove to be of general utility. | ||
| Scheme 3 | ||
Molecular aggregates
The construction of molecular aggregates, based on supramolecular host–guest chemistry is a burgeoning area of research. Work carried out by Colin Raston and his group at Monash University. Australia, and latterly at Leeds University, has demonstrated such chemistry (Eur. J. Org. Chem., 2001, 3227) based on green synthetic procedures for the construction of the building blocks. They have shown that inclusion of guest molecules can take place inside molecular capsules based on terpyridines and calixarenes, prepared using solvent-free syntheses, avoiding the more wasteful routes originally employed.Ionic liquids and supercritical fluids
Ionic liquids and supercritical fluids represent two classes of solvents possessing excellent properties for green processing. A very innovative combination of these two solvents has led to a very elegant methodology for hydrovinylation reactions. Hydro- vinylation of alkanes is an addition reaction leading to new alkenes, and can be carried out enantioselectively using Wilkes catalysts, a nickel-based catalyst where the nickel is coordinated to a chiral phosphorus centre. Walter Leitner of the Max-Planck Institut in Mulheim, and Peter Wasserscheid of the RWTH Aachen have joined forces to develop a continuous process based on this reaction in ionic liquids and compressed CO2 (Angew. Chem., Int. Ed., 2001, 40, 2697). They have immobilised an ionic version of Wilkes catalyst by making it the counterion of an ionic liquid, and carried out a continuous hydrovinylation and separation using compressed CO2 as the mobile phase, simultaneously reducing the viscosity of the system and improving mass transport. Conversions and ee values were good and dropped only slightly over tens of hours of processing.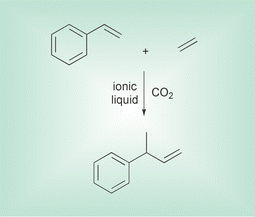 | ||
| Scheme 4 | ||
Carbonylations
Pd-catalysed carbonylation reactions are a versatile route to a range of acid derivatives, but are less well developed than other C–C bond forming analogues such as the Heck and Suzuki coupling reactions. Matthias Beller and co-workers from the University of Rostock have now published details of an exciting new catalyst system which allows the highly efficient carbonylation of aryl chlorides, the cheapest and most readily available class of aryl halides (Angew. Chem., Int. Ed., 2001, 40, 2856). They have shown that monodentate phosphines do not produce catalysts with acceptable activity in the carbonylation of aryl chlorides, but bidentate phosphines are excellent in this respect. After screening some ferrocenyl phosphines (a well-developed class of diphosphines) they found that the diphosphine shown was an excellent catalyst, and carbonylations could be carried out in essentially quantitative yield and with selectivities often >90%. They showed that the carbonylated intermediate could be trapped with alcohols, water and amines, leading to esters, acids and amides, all in high yield. | ||
| Scheme 5 | ||
Tertiary C9–C11 carboxylic acids are used in a variety of applications such as coatings. They are produced by carbonylation of alkenes using extremely acidic liquids. The search for solid acids capable of carrying out this transformation has met with limited success, but Jean-Paul Lange of Shell, Amsterdam, and Leo Petrus of Resolution Performance Products (Amsterdam) have published details of their investigations into this reaction (Appl. Catal. A, 2001, 216, 285). They found that under optimal conditions, sulfonated resins such as Nafion and Amberlyte resins were able to give good yields of acid using the product as solvent, and a relatively low concentration of reactants under CO pressure and temperatures of ca. 150 °C. Interestingly inorganic solid acids were not effective.
 | ||
| Scheme 6 | ||
Heterogeneous catalysis
A further use of ionic liquids relies on the potential to complex a Lewis acid to the counterion to provide an ionic liquid solution of Lewis acid, which can then be used to catalyse for example the Friedel–Crafts reaction. Analogous complexes can also be immobilised on silica and used as heterogeneous catalysts. The group led by Wolfgang Hölderich at the RWTH in Aachen has published details of such catalysts based on imidazolium chloride complexes of ferric chloride (Appl. Catal. A, 2001, 215, 185). These are excellent catalysts for the acylation of anisole and alkylbenzenes but the heterogeneous catalysts are subject to leaching of the iron chloride into solution. Reactions carried out in a gas phase flow reactor were also attempted, and showed interesting results, although some loss of acetyl chloride was observed. Nonetheless these materials are genuinely catalytic in this reaction type. | ||
| Scheme 7 | ||
Oxidations
Selective oxidations using oxygen are one of the most important technologies available to green chemistry. However, the direct activation of oxygen without adjuncts such as bromide or aldehydes is often difficult. Laurent Gaillon and Fethi Bedioui of the CNRS in Paris have now demonstrated the potential of electrochemical activation of oxygen via Jacobsen’s catalyst in ionic liquids (Chem. Commun., 2001, 1458). They have shown that the key steps in the activation of oxygen can be achieved using carbon electrodes, indicating that this method may be able to activate oxygen, and transfer it to an olefin selectively without the need for other auxiliary reagents. | ||
| Scheme 8 | ||
A contribution to the oxidation of alkanes by hydrogen peroxide has appeared from Georg Süss-Fink and co-workers from Neuchajtel in Switzerland and Moscow. They have shown that a range of alkanes can be oxidised to the hydroperoxide (and then subsequently decomposed to the alcohol and ketone) with hydrogen peroxide using vanadium-containing polyphosphomolybdates (Appl. Catal. A, 2001, 217, 111). Using this system in acetonitrile at moderate temperatures, they achieved good turnover numbers and high yields (>30%) in the case of cyclooctane, n-octane, adamantane and even ethane. Only traces of methane oxidation could be observed, and in this case solvent breakdown was observed.
 | ||
| Scheme 9 | ||
Another paper dealing with the oxidation of hydrocarbons has been published by the group of Laureano Canoira on the Polytechnic University of Madrid (Appl. Catal. A, 2001, 218, 269). The reaction investigated here is the nickel-catalysed oxidation of ethylbenzene. The group had previously found that quaternary ammonium salts are excellent co-catalysts for the production of ethylbenzene hydroperoxide (an intermediate in the synthesis of phenethyl alcohol, acetophenone and styrene) using soluble Ni(acac)2 complexes and oxygen. They now present results which indicate that the use of 1-butyl-3-methylimidazolium hexafluorophosphate gives better results than the simpler tetraalkylammonium salts used up to now.
 | ||
| Scheme 10 | ||
Phase-transfer catalysis
The use of phase-transfer catalysis can often be a very attractive synthetic protocol, but one which is made much less attractive by the catalysts’ inherently good solubility in both aqueous and organic phases. This property make the catalysts difficult to recover, reducing its attractiveness industrially and environmentally. The group led by Sathinder Luthra from Imperial College, London, has now published details of a potential breakthrough in this respect (Chem. Commun., 2001, 1468). They have found that nanofiltration membranes can be successfully used to recover phase-transfer catalysts from a toluene/water mixture after reaction. Catalyst recovery was excellent and reuse was demonstrated over three cycles. Some fouling of the membrane was noted, but was easily reversible.Centre for Green Chemistry, Monash University
A report on the official opening
The new facilities of the Australian Research Council (ARC), Special Research Centre (SRC), the Centre for Green Chemistry were officially opened by Senator Kay Patterson on Monday 9th July 2001. Among the 80 or so guests was Professor Colin Raston, whose enthusiasm led to the establishment of the Centre, but who has since moved to a chair at Leeds University, UK. Other distinguished guests included Dr Denny Hjeresen, Director of the American Green Chemical Institute, Professor Pietro Tundo, Director of the European based Inter-University Consortium for Chemistry and the Environment and a number of visitors from the UK, China, Portugal, Italy, South Africa, Sweden and Australia. | ||
| Fig. 4 | ||
The Centre for Green Chemistry (CGC) commenced operation in January 2000 and has grown rapidly since its inception. There are now more than 24 Ph.D. students and visiting scholars, 10 postdoctoral research fellows and 4 staff within the Centre and a large number of associated researchers from diverse scientific backgrounds, working on green chemistry research projects. These include a number of faculty from the School of Chemistry as well as Monash University Departments of Materials Engineering, Biochemistry, Earth Sciences, (and it is hoped soon Chemical Engineering); faculty at other universities in Australia and researchers from divisions of CSIRO including Forestry and Forestry Products, Minerals, Molecular Sciences and Manufacturing Science. International collaborations with scientists in the UK, USA, Japan, Sweden and South Africa are well developed and expected to grow from strength to strength and other opportunities for extending links are being actively explored, as are possibilities for student exchange.
ARC Special Research Centres in contextThe Australian Research Council (ARC) is the main funding agency in Australia for basic research. It supports research in essentially all fields from science, engineering and new technologies through to social sciences, humanities and the creative arts (except clinical medicine and dentistry). Special Research Centres are funded by the Australian Federal Government via the Australian Research Council and are designed to ‘enable special concentrations of staff and resources for research and research training of a longer term nature’. The Centres are funded for up to nine years, with reviews every three years. Eleven new Centres were identified from 76 applications to receive funding beginning in 2000. AU$135 million funding is allocated for these Centres over their lifetime. |
The purpose of the Special Research Centre is to engage in fundamental research and development towards the design, manufacture and use of clean chemical processes that have little or no pollution potential or environmental risk and are both economically and technologically feasible. Clearly, interaction with Australian industry is of paramount importance and collaborations with companies representing industries as apparently diverse as mining, pulp and paper processing and pharmaceutical development are in place, whilst others are being explored. Evidence that Australian industry is actively exploring the concepts of sustainability and green chemistry comes from a number of invitations to address company boards and future planning workshops received by Centre representatives.
 | ||
| Fig. 5 Part of the Centre for Green Chemistry Laboratories nearing completion early in 2001. The laboratories are no longer quite this spotless but now buzz with productive activity. | ||
The CGC aims to provide a scientific base for future chemical technology, identifying niche areas in the Australian context and beyond for global initiatives, as well as providing valuable training of future workers in this area through postgraduate scholarships and postdoctoral fellowships. It will provide a common focus, build up a critical mass of researchers, and provide the synergy and creativity expected of a SRC. In addition, the CGC must work to become financially independent to ensure its continued existence beyond the period of the ARC funding.
To meet these aims within the timeframe allowed, four main research streams have been identified to provide a suitable mix of:
• fundamental research
• strategic research with unencumbered intellectual property
• research for the public good
• industrial collaborations
This is designed to provide a sustainable mix of high-profile fundamental scientific research, new opportunities and income-generating segments with the goal of providing the best possible research training for students and fulfilling the mission statement with regard to ‘becoming internationally recognised for research and teaching in the field of green chemistry’.
Current projects may be broadly divided into 3 groups:
• Those associated with the design and synthesis of more benign products such as: Novel Boron-based Wood Preservatives; Non-addictive Opioid Analgesics; Supramolecular Polymers; Soil Remediation Products and Green Corrosion Inhibitors (for use in recycling water systems or with replacement of toxic reagents); Benign Mineral Processing Methods; Alternative Routes to Isocyanates (phosgene replacement)
• Those centred around the development of green synthetic protocols and enabling methodologies: Understanding Solvent-free Reactions and Supramolecular Reaction Control; Microwave Heating and High-Temperature Aqueous Reactions; Green Nucleophilic Addition and Ring Closure (NARC) reactions; Green Medicinal Chemistry (simultaneous development of new therapeutic agents and clean synthetic routes); Electrochemical Methodologies; Use of Ionic Liquids as Green Reaction Media and a number of projects relating to catalysis. Many of these projects are fundamental studies leading to a greater understanding of the science underlying the technologies.
• Projects with the goal of facilitating the use of renewable raw materials: FIA/FTIR/Raman methods for of real-time monitoring of chemicals in fermentation processes; Biotechnology in Green Chemistry and the Development of Drug Products from Plant-Derived Chemicals.
While Special Research Centre funding is granted for the purpose of development of research in a specific field, education in green chemistry is also considered to be an important part of the Centre’s role and a number of programmes are being developed. A course in green chemistry is offered at 3rd year level at Monash University and green chemistry laboratory exercises are incorporated into the practical component of the final year courses. Postgraduate courses are under development and a part time Community Outreach Officer has recently been employed to increase the capacity of the Centre to offer lectures to schools and community groups. Interactions with secondary school students and teachers are deemed especially important as, in common with many other countries, enrolment in science courses at tertiary level shows a downward trend. Exciting lectures, which demonstrate the application and importance of green chemistry by way of everyday examples and chemical demonstrations, are under development and will be delivered by the Community Outreach Officer at individual schools. Expanded versions of these lectures will be presented this year as the Hartung Youth Lectures and Tasmanian Youth Lectures, sponsored by the Royal Australian Chemical Institute (RACI). Teachers from the Science Teachers Association of Victoria (STAV) and the Chemical Education Association (CEA) are collaborating with key researchers in the Centre for Green Chemistry to develop resources to aid teachers who wish to incorporate green chemistry into their high school courses.
 | ||
| Fig. 6 Dr Ulf Kreher, a postdoctoral fellow, assembling the reaction vessel of the custom designed microwave batch reactor which allows continual monitoring of temperature and pressure, removal of samples for monitoring and rapid well-controlled cooling via a cold-finger. | ||
 | ||
| Fig. 7 Distinguished guests at the official opening of the new facility of the Centre for Green Chemistry. From left, Professor W. Roy Jackson, Director, Centre for Green Chemistry; Dr Geoff Knights, Chair, Centre for Green Chemistry Advisory Board; Hon. Kay Patterson, Senator for Victoria; Mr Jerry Ellis, Chancellor, Monash University. | ||
As the Centre expands further and develops both its educational, research and industrial projects the challenge will be to maintain focus and ensure that the strategic goals of the Centre are met whilst remaining flexible and open to innovation. Sound science, a commitment to education and close relationships with all the players in this developing field will ensure the future of this exciting project.
For further information see the Centre for Green Chemistry website: http://web.chem.monash.edu.au/ GreenChem/ and to receive electronic copies of the Newsletter send an e-mail to Green.Chemistry@sci.monash.edu.au.
From plant to plastic,
Renewables are fantastic!
Elinor Scott and her colleagues at ATO describe their research in the area of polymers and plastics aims at integrating health, safety and environmental aspects with a high product performance and economic sustainability. ATO continues to be naturally inspired in order to explore the synergy between resources, materials and technologies from synthetic and natural origin.
| ATO (The Agrotechnological Research Institute) in Wageningen is situated in the ‘green valley’ of Dutch agricultural innovation. As one of its activities, ATO successfully performs developments in collaboration with industry leading to the future of renewable resources in non-food markets.See http://www.ato.wageningen-ur.nl/ |
Introduction
The large demand for petrochemical- based polymers and plastics has led to optimized product chains in terms of the economics and performance of the product, as well as in aspects such as process optimization and environmental impact. However, there is a current resurgence of interest in sustainable natural products for the preparation of alternative plasticisers, alternatives to diols, diacids and aromatic molecules. It is proposed that biosynthetic systems can be thought of as a useful (bio-) chemical and technological toolbox. For example, biological reactions may lead to more efficient routes to materials or allow incorporation of a naturally occurring chemical structure, which offers better functionality of the material.Considerations such as resource sustainability, (bio-) chemistry, engineering technology and environmental impact are given special attention. From a chemical or technological perspective, by adopting a combined biosynthetic methodology and petrochemical technology approach there is an important complementary and synergistic effect. Therefore materials can be designed with a desired composition, structure and property and prepared by an effective, energy-efficient and safe method.
 | ||
| Scheme 12 | ||
Aspects of monomer, polymer and wood additives, biopolymer technology and post-consumer life research are described below.
(Bio-) chemical monomer and polymer synthesis
Recent advances in combining conventional and novel (bio-)chemical transformations and use of sustainable resources, make it possible to enter existing product chains with chemicals prepared from a sustainable natural origin. One such entrance point is the synthesis of chemical building blocks for the preparation of polymers. These building blocks may have:• Existing functionality
• Alternative functionality, characteristics similar to traditional petrochemically derived aromatic monomers, e.g. terephthalic acid.
 | ||
| Scheme 13 | ||
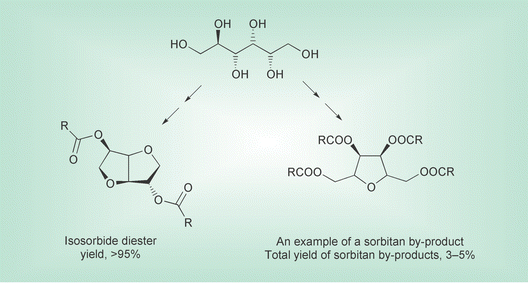
These compounds, for example, have rigid structures and can undergo polycondensation reactions to produce polyesters with specific properties. As well as this, isosorbide has been esterified and behaves as an effective plasticiser. Other activities in (bio-) chemical monomer and polymer synthesis include fermentation, biocatalysis and protein engineering.
 | ||
| Scheme 14 | ||
Polymer additives—plasticisers
Many conventional plasticisers are based on phthalate esters.16,17 Currently there is concern over the use of phthalates as certain derivatives are suspected of being carcinogens or endocrine disrupters. Environmental studies have shown that they can be found in the food chain, in sediments and water supplies.18 Most are monomeric and eventually, depending on conditions, migrate out of the polymer. These problems may be overcome using polymeric plasticisers, but these tend to have low efficiency and high costs.17 Some monomeric non-toxic alternatives for phthalates such as citrates, benzoates and adipates are currently commercially available, but with some limitations for widespread use.17The use of isosorbide diesters as plasticisers was first mentioned as early as 1953, but limited yields and high price of isosorbide proved detrimental to further development.19 At ATO isosorbide plasticisers have been developed using a new method.5 Isosorbide diesters show similar plasticising behaviour to phthalate (esters) and are compatible with poly(vinyl chloride) over a wide concentration range.
Recently a one-pot procedure to prepare isosorbide diesters, from inexpensive sorbitol, has been developed. The proprietary technology uses a specific catalyst making it possible to do in-situ dehydration of sorbitol and esterification of isosorbide using the alkanoic acid as the solvent. By careful control of some reaction parameters sorbitan byproducts are minimised.
One-pot procedure
Currently the reaction mechanism is being investigated to determine the influence of reaction parameters on product distribution. These compounds are currently undergoing an extensive testing program.Other polymer additives being developed include:
• Halogen-free flame retardant chemicals for electric cables and equipment.
• Antioxidants of natural origin for use in polymers
Wood—flame retardants
Wood has many properties which makes it a very useful building material, however its flammability can be a disadvantage in some applications. To reduce flammability, chemicals, e.g. phosphorus compounds, can be impregnated to reduce the temperature of the pyrolysis and form a protective char layer.21 However, current flame-retardant chemicals have some negative aspects. For example under the influence of humidity they tend to slowly migrate out of the material and they increase the level of water absorption causing faster degradation of the wood due to fungal growth. In co-operation with industrial partners22 a project on development of more environmentally friendly flame retardants has recently started. The use of these new compounds combines both flame retardancy and durability of wood or wood-based products (Fig. 1). | ||
| Fig. 8 Untreated wood (2× left) and newly developed treated wood (2× right) after flame test. | ||
In some building situations, e.g. escape routes, the use of wood may not meet the required levels required for flame retardancy. When the wood materials can be modified to meet these requirements, there becomes a possibility of using wood-based products.
Within this project chemicals, which are directly bonded on to the reactive groups of wood, and treatments, are being developed. As well as this, the wood also becomes intrinsically more hydrophobic and so a more resilient and durable product can be made. An additional benefit is the swelling of the wood may be reduced—an important feature in construction materials. Presently there are a series of compounds and treatments with promising results.
Biopolymer products—starch-based packaging
Polymers from natural resources are finding more and more applications in various markets. Food packaging is a potential market for these bio-based polymers. The aim of this research is to show that it is possible to produce packaging materials totally from bio-based polymers that do not only meet the requirements of the food packaging industry, but also give additional advantages over conventional packaging materials. The properties of starch make it a suitable alternative to conventional packaging materials. However, there are some aspects of using starch which need to be overcome. For example the water sensitivity of starch may limit the application and should be improved.To overcome this problem, an approach has been to chosen where starch is coated with (biodegradable) polyesters. While this approach is useful in improving water resistance, the adhesion between the surfaces of the starch and the polyester in the layered foil is a very important aspect in developing a durable product. This has been a focus in the research programs. One successful approach to reduce the water sensitivity and improve adhesion has been the development of a co-extruded multi-layered film (Fig. 2).
 | ||
| Fig. 9 Film blowing of co-extruded biopolymer multi-layered films. | ||
The performance of these barrier laminate films has been evaluated in real life packaging tests. Results show that the developed packagings have a positive effect on the shelf life of Modified Atmosphere (MA)-packed cut vegetables and fresh salads when compared to conventional packaging materials. Little to no negative influence on the product quality during the shelf life of the packed products was observed in MA packaging of fresh bread rolls, meat and cheese.
Other activities in the biopolymer area include:
• Biosynthetic polymer synthesis
• Foam and injection moulding technology of biopolymers
• Product development with compostable polymers
Post-consumer product life
The test below (Fig. 3) simulates conditions in a municipal solid waste treatment plant. This is just one on the many conditions that are studied to understand the relationships between polymer structure and (bio-)degradation behavior. Insight in to these relationships can then be used to predict degradation of newly developed materials and provides a tool for selectively engineered degradable products. At ATO research has been performed to improve existing tests and develop new tests to assess biodegradation, mineralisation and disintegration of products under specific (simulated) environmental conditions. | ||
| Fig. 10 Laboratory composting test. | ||
Other activities in post-consumer product studies include additives and technology to aid recycling.
E. Scott, R. van Tuil, D. van Es, R. Bezemer, G. Schennink and M. van der Zee are based at ATO, PO Box 17, 6700 AA Wageningen, The Netherlands Tel: +31 317 475029.
Acknowledgements
Stephan Hulleman and his group Polymers, Composites and Additives. Industrial partners and funding bodies that participate in the work.References
- R. Gächter and H. Müller, Plastic Additives Handbook, 4th edn.; Carl Hanser Verlag, Munich, 1993. Search PubMed.
- A. S. Wilson, Plasticisers; Principles and Practice, Cambridge, The Institute of Materials, 1995. Search PubMed.
- Reports of the National Toxicology Program (U.S. Dept. of Health and Human Services) by the Center for the Evaluation of Risks to Human Reproduction (CERHR): http://cerhr.niehs.nih.gov.
- I. Hayashi, S. Hamada and Y. Hachihama, Kogyo Kagaku Zasshi, 1953, 56, 623–625..
- J. C. Jansen and H. Luitjes, Bicyclooctane derivatives as plasticisers; Inst. voor Agrotechnologisch Onderzoek, EP1058711, 1998. Search PubMed.
- W. D. Ellis and R. M. Rowell, Wood Fiber Sci., 1989, 21(4), 367–375 Search PubMed.
- In co-operation with SHR, Houtbedrijf Van Hout b.v., Kegro Deuren b.v., Heraklith b.v., IVAM Environmental Research b.v., Wageningen UR and Dick Peters b.v..
| This journal is © The Royal Society of Chemistry 2001 |
