2001 Green Chemistry Challenge Awards
The following are this year’s recipients of the major US awards in
green chemistry
European teaching on renewable resources
Towards a metabolic society: a thermodynamic view
A greener future with biodiesel
Chemical companies take energy management to heart
2001 Green Chemistry Challenge Awards
The following are this year’s recipients of the major US awards in
green chemistry
Academic category
Professor Chao-Jun Li, Tulane University, was selected for designing a wide variety of transition metal mediated and catalyzed reactions that can be accomplished in air and water. Traditionally, these reactions have been carried out in an organic solvent under an inert atmosphere. Li has developed a novel [3 + 2] cycloaddition reaction to generate 5-membered carbocycles in water; a synthesis of beta-hydroxyl esters in water; a chemoselective allylation and pinacol coupling reaction mediated by manganese in water; and a novel alkylation of 1,3-dicarbonyl type compounds in water. In addition, a number of Barbier–Grignard type reactions in water have been developed. Water offers many advantages as a solvent. Water is readily available and inexpensive, and is not flammable, explosive, or toxic.Protection/deprotection steps can be avoided, and products may be isolated by simple phase separation rather than by energy-intensive and organic-emitting processes involving distillation of organic solvent. Several reactions also demonstrate unprecedented chemoselectivity that eliminates byproduct formation and separation. The open-air feature offers convenience in operations of chemical synthesis involving small-scale combinatorial synthesis, large-scale manufacturing, and catalyst recycling. The catalytic reactions developed by Professor Li have widespread applications in the synthesis of pharmaceuticals, fine chemicals, petrochemicals, agricultural chemicals, polymers and plastics. Through his development of transition metal mediated and catalyzed reactions in air and water, Professor Li provides an attractive alternative to the inert atmosphere and organic solvents traditionally used in many synthetic reactions. For more information on the work of Professor Li see http://www.tulane.edu/∼ chemistry/ Li.html
Small business category
EDEN Bioscience Corporation was selected for developing harpin technology that, when applied to crops, increases plant biomass, photosynthesis, nutrient uptake and root development, and ultimately leads to greater crop yield and quality. Harpins are nontoxic, naturally occurring proteins that trigger a plant’s natural defence systems to protect against disease and pests and simultaneously activate certain plant growth systems without altering the plant’s DNA. EDEN discovered that once a harpin protein binds to a receptor molecule on the plant surface, several biochemical events are induced. First, production of hydrogen peroxide, an important mechanism of plant defense, is induced in plant cells, followed by stimulation of a series of ion exchanges in the cell membrane. A series of signal transduction events leads to several beneficial plant responses. The result of this technology is an EPA- approved product called Messenger®, that has been demonstrated on more than 40 crops to effectively stimulate plants to defend themselves against a broad spectrum of viral, fungal, and bacterial diseases, including some for which there currently is no effective treatment. In addition, Messenger® has been shown through an extensive safety evaluation to have virtually no adverse effect on any of the organisms tested, including mammals, birds, honey bees, plants, fish, aquatic invertebrates and algae. In addition, harpin-based products are produced in a water-based fermentation system that uses no harsh solvents or reagents, requires only modest energy inputs, and generates no hazardous chemical wastes. As with most proteins, harpin is a fragile molecule that is degraded rapidly by UV and natural microorganisms and has no potential to bioaccumulate or to contaminate surface or groundwater resources. Using environmentally benign harpin protein technology, growers for the first time in the history of modern agriculture will be able to harness the innate defence and growth systems of crops to substantially enhance yields, improve crop quality, and reduce reliance on conventional agricultural chemicals. For more information on Eden Biosciences and Messenger® see http://www.edenbio.comAlternative synthetic pathways
Bayer Corporation and Bayer AG were selected for their synthesis of a biodegradable chelating agent. Most classic chelating agents are poorly biodegradable; some are quite persistent and have been detected in the surface waters of rivers and lakes and in make-up water processed for drinking water. Bayer Corporation manufactures a readily biodegradable and environmentally friendly chelating agent, DL-aspartic-N-(1,2-dicarboxyethyl) tetrasodium salt, an aminocarboxylate also known as sodium iminodisuccinate. This agent is characterized by excellent chelation capabilities, especially for iron(III), copper(II), and calcium, and is both readily biodegradable and benign from a toxicological and ecotoxicological standpoint. It is also produced by a 100% waste-free and environmentally friendly manufacturing process. Nearly all aminocarboxylates in use today are acetic acid derivatives produced from amines, formaldehyde, sodium hydroxide, and hydrogen cyanide. The industrial use of thousands of tons of hydrogen cyanide is an extreme toxicity hazard. In contrast, Bayer’s sodium iminodisuccinate is produced from maleic anhydride (a raw material also produced by Bayer), water, sodium hydroxide, and ammonia. The only solvent used in the production process is water, and the only side product formed, ammonia dissolved in water, is recycled back into sodium iminodisuccinate production or used in other Bayer processes. Because sodium iminodisuccinate is a readily biodegradable, non-toxic, and non-polluting alternative to other chelating agents, it can be used in a variety of applications that employ chelating agents, including detergents, agricultural nutrients, and household and industrial cleaners. For more information see http://www.bayerus.com and http://www.bayer.deAlternative reaction conditions
Novozymes North America, Inc., was selected for designing an enzymatic process for treating cotton textiles that provides an economical and environmentally friendly alternative to alkaline scour systems currently used in the textile industry today. Conventional chemical preparation processes involve treatment of the cotton substrate with hot solutions of sodium hydroxide, chelating agents, and surface active agents, often followed by a neutralization step with acetic acid. This series of various treatments and rinsing steps generates large volumes of wastes, including large amounts of salts, acids, and alkali. The BioPreparation™ technology developed by Novozymes is an alternative to sodium hydroxide that offers many advantages for textile wet processing, including reduced BOD/COD, and decreased water use. Pectate lyase is the main scouring agent that degrades pectin to release the entangled waxes and other contaminants from the cotton surface. This enzyme is also compatible with other enzymatic preparations (amylases, cellulases) used to improve the performance properties of cotton fabrics. Because BioPreparation™ uses fewer chemicals and rinsing steps than required during a traditional caustic scour, textile mills may save as much as 30–50% in water costs by replacing caustic scours or by combining the usually separate scouring and dyeing steps into one. A recent statistical survey determined that 162 knitting mills used 89 million m3 per year of water in processing goods from scouring to finishing; the BioPreparation™ approach would save from 27 to 45 million m3 per year of water. In addition, field trials established that BOD and COD loads are decreased by 25 and 40%, respectively, when compared to conventional sodium hydroxide treatments. Furthermore, costs savings of 30% or more per mill can be realized. For more information on Novozymes North America and BioPreparation® see http://www.novozymes.comDesigning safer chemicals
PPG Industries was selected for its discovery that yttrium can be used as a substitute for lead in cationic electrocoatings without any sacrifice in corrosion performance. Although much less studied that lead, the available data on yttrium indicate orders of magnitude lower hazard. As a dust hazard, yttrium is 100 times safer than lead at typical levels of use. In electrocoat applications, yttrium is twice as effective as lead on a weight basis, allowing the formulation of commercial coatings that contain half the yttrium by weight relative to lead in comparably performing lead-containing products. It has been found that yttrium is deposited as the hydroxide in an electrocoat, then converted to the oxide during normal baking of the electrocoat. The oxide is extraordinarily non-toxic by ingestion as indicated by the LD50 of >10 g kg–1 in rats, which is in stark contrast to lead. The ubiquitous nature of yttrium in the environment and the insoluble ceramic-like nature of the oxide combine to make it an unlikely cause of future environmental or health problems. An environmental side benefit of yttrium is its performance over low nickel and chrome-free metal pretreatments. By using yttrium in the electrocoat step, chrome can be completely eliminated using standard chrome-free rinses and low nickel or possibly nickel-free pretreatments, both of which are commercially available today. For PPG pretreatment customers, this should result in the elimination of up to 25,000 lb of chrome and 50,000 lb of nickel (annually) from PPG products. As PPG customers implement yttrium over the next several years, approximately one million lb of lead (as lead metal) will be removed from the electrocoat applications of PPG automotive customers. For more information on PPG Industries and their electrocoatings see http://www.ppg.coHighlightsDuncan Macquarrie reviews the latest research in green chemistry
Imino-aldol reactions
The imino-aldol reaction is a very useful method for the formation of amino acid derivatives. Obviously, enantioselective versions of this reaction are vitally important in the development of novel amino acids, and chiral amines. William Wulff and co-workers from Michigan State University, USA, have recently published work relating to two different ligand systems for the zirconium- catalysed reaction of imines with silyl acetals (Angew. Chem., Int. Ed., 2001, 40, 2271).Their work involves the use of 6,6-dibromo-1,1’:2,2’-binaphthyl (diBrBINOL) and the related VAPOL ligands, which form 2:1 complexes with Zr(OtBu)4. These complexes allow high levels of enantioselection to be obtained, with the VAPOL system yielding ee’s of >98% even at 100 oC in some cases.
 | ||
| Scheme 1 | ||
Ethylbenzene → styrene
One of the most important industrial processes is the oxidative dehydrogenation of ethylbenzene to styrene. Currently K-promoted iron catalysts are used in this process, which run at temperatures around 900 K. The process is thus energy intensive, and novel catalyst systems which can reduce the energy burden are welcome. Robert Schlögl and his group at the Max Planck Institute in Berlin, Germany, have published a new catalyst based on carbon nanofilaments, which allows the reaction to proceed efficiently at 830 K (Angew. Chem., Int. Ed., 2001, 40, 2066). Their initial idea came from the potential role of carbon deposits on various catalysts, and was developed by examining a series of carbons. They found that nanofilaments of carbon were both the most active and the most stable under the oxidative reaction conditions, something that precludes most carbon sources. Under their conditions, excellent activity could be sustained for several hours, indicating considerable promise for this new material. | ||
| Scheme 2 | ||
Polymerisation
The production of resins based on petrochemical feedstocks is a major area of polymerisation technology. James Clark and his group at York University, UK, have developed a heterogeneous AlCl3-based catalyst system which is very effective at polymerising these monomer mixes, and avoids the need for an aqueous quench reaction to remove the catalyst (Org. Proc. Res. Dev., 2001, 5, 249). Their catalyst is also very effective at increasing polymer yield and importantly, controlling the molecular weight distribution, critical to the successful application of the polymer. | ||
| Scheme 3 | ||
Immobilised Lewis acids
An interesting route to immobilised Lewis acids has been published by the group of M. V. Landau at the Ben Gurion University in Beer-Sheva, Israel (Chem. Commun., 2001, 992). Their approach utilises quaternary ammonium species immobilised on mesoporous high surface area silicas. These are present in the form of chlorides, and it is the chloride which behaves as a Lewis base to complex the acidic component. They have shown that quaternary supported tin tetrachloride is active in the Prins condensation of formaldehyde and isobutene to isoprenol. Interestingly, it is significantly more active than the same Lewis acid directly adsorbed on the silica support. This may provide a useful general method for the immobilisation of Lewis acid, complementary to the direct attachment route. | ||
| Scheme 4 | ||
Arylamines and diarylamines
The synthesis of arylmines and diarylamines is required for the synthesis of many bioactive compounds, as well as antioxidants (see Green Chem., 1999, 1, G41 for an alternative clean synthesis of a diarylamine). Jon Antilla and Stephen Buchwald at Massachusetts Institute of Technology (MIT), USA, have now developed a method for the coupling of amines with arylboronic acids, which relies on a copper acetate catalyst (Org. Lett., 2001, 3, 2077). This reaction proceeds under air, which appears to be vital for the re-oxidation of the copper, and myristic acid, which helps to solubilise the catalyst system. Even under non-optimal conditions for oxygen transport, the reaction proceeds very well, giving high yields of product. Functional group tolerance is excellent, and appears to be better than with Pd-based couplings, with amides and alcohols reacting smoothly. Aliphatic amines also give reasonable results. | ||
| Scheme 5 | ||
Enzymes
Immobilised enzymes represent an interesting class of asymmetric catalysts, combining the advantages of an efficient reaction under mild conditions with the ease of separation of heterogeneous catalysts (in optimally designed systems). The group led by Harald Gröger at Degussa and the Institut für Technoligie und Biosystemtechnik in Braunschweig, Germany, have published details of such a system, consisting of (R)-oxynitrilase entrapped in a poly(vinyl alcohol) matrix (Org. Lett., 2001, 3, 1969). They have found that this immobilised enzyme can catalyse the addition of HCN to benzaldehyde in excellent yield and ee to give (R)-mandelonitrile at room temperature and slightly acidic pH. Yields in the best solvent system were higher than with free enzyme, and ee’s were always above 90%, some as high as 99%. Leaching was not found, and the catalyst could be recovered and recycled.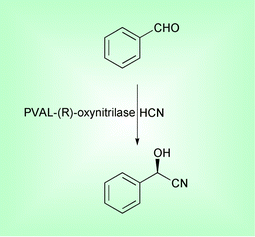 | ||
| Scheme 6 | ||
A further interesting paper on enzyme-catalysed reactions comes from Keichi Watanabe and Shin-ichi Ueji from Kobe University, Japan (J. Chem. Soc., Perkin Trans. 1, 2001, 1386). They have found that the simple addition of around 50% DMSO to the aqueous buffer system has an enormous positive effect on the enantioselectivity. For example, the lipase-catalysed hydrolysis of various phenoxypropionates gives an enantiomeric excess of 25–49%, depending on the structures, when no DMSO is present. Addition of DMSO to the reaction medium causes the ee to rise to 100%, at the cost of a slower reaction (ca. 5–6 times longer reaction time). Various lipases gave similar effects.
 | ||
| Scheme 7 | ||
Heck reactions
Heterogeneous Heck catalysts continue to be of interest. Laurent Djakovitch and Klaus Koehler of the Technishce Universität in Munich, Germany, have now described a Pd–NaY zeolite catalyst which shows excellent activity in the coupling of aryl halides with alkenes (J. Am. Chem. Soc., 2001, 123, 5990). They exchanged Na–Y zeolites with Pd species and then used them in the Heck reaction of a series of reaction partners. Their results indicated that the catalyst was highly active, converting activated substrates rapidly and in high yields, and even showing some activity for aryl chlorides. The catalysts could be recovered and reused with almost identical activity. No leached Pd could be detected. | ||
| Scheme 8 | ||
Functionalisation of silicones
Functional silicones are interesting materials for a wide range of applications, including advanced electronic and optical devices. However, the functionalisation of simple polysiloxanes, carried out by oxidative addition of the Si–H groups on the backbone to alcohol groups, is plagued by problems of selectivity—several side-products are formed, and control over the exact nature of the product is difficult. Philip Boudjouk and his group at the Center for Main Group Chemistry at North Dakota State University, USA, have now provided an effective Rh catalysed route to these important materials (Organometallics, 2001, 20, 2725). They used RhCl(PPh3)2 to effect the alcoholysis of the Si–H bond with a range of alcohols, leading to high levels of functionalisation, without the problems of crosslinking and isomerisation generally seen with existing systems. Many different functionalities can be introduced, making the method versatile. The only solvent used in the paper was d6-benzene, as the reactions were all monitored in situ by NMR, but it seems likely that other solvents could easily replace benzene in a more practical system. | ||
| Scheme 9 | ||
Cascade reactions
Cascade reactions are making a significant impact in organic synthesis, due to their advantages of minimising the number of isolation stages that have to be carried out, simplifying multi-step synthetic strategies, and eliminating wasteful purification of intermediates. Andrew McCorrel and John Walton of St Andrews University, UK, have published an excellent review on radical cascade processes (Angew. Chem., Int. Ed., 2001, 40, 2224) in which they discuss a wide range of radical based one-pot multistep synthetic methods, many of which have potential for minimising environmental impact. | ||
| Scheme 10 | ||
Supramolecular assemblies
Supramolecular assemblies with defined architecture are proving to be very versatile and popular in the field of new materials and nanotechnology. While the vast majority of the materials prepared are derived from petroleum-based feedstocks, relatively little has been done in the field of renewable raw materials in this are. The group led by George John and Toshimi Shimizu of the CREST of Japanese Science and Technology and the AIST in Ibaraki, Japan, has now published a route to nanotubes based on cardanol, a product derived from cashew nuts (Adv. Mater., 2001, 13, 715). A mixture of four glycosides (the one shown and the corrsponding diene, monoene and saturated equivalents, saturation beginning from the terminal double bond and progressing towards the aryl group) were dissolved in hot water and allowed to cool. This produced supramolecular assemblies, with the pure saturated glycoside giving nanotubes. Such nanotubes could have a range of applications in catalysis, electronics and separation technology amongst others. | ||
| Scheme 11 | ||
Alkene hydroformylation
Hydroformylation of alkenes is a key process for the functionalisation of alkenes. Biphasic systems with water soluble metal complexes have shown promise, but have some drawbacks, especially in the case of internal alkenes. Progress in this respect has recently been described by Shoichi Shimizu and co-workers from Nihon University in Chiba, Japan (New J. Chem., 2001, 25, 777). They used a calixarene-based ligand system for a rhodium complex as a water-soluble catalyst, and found that excellent conversions could be attained over several cycles, overcoming the drawbacks seen with other methods. Isomerisation of the alkene double bond leads to all four possible aldehydes from trans-4-octene, although the quantity of the most desirable product, the terminal aldehyde does not exceed 24%; work is ongoing to improve this. | ||
| Scheme 12 | ||
Diels–Alder reactions
The combination of ionic liquids and scandium salts has been found to be a very powerful method for the Diels–Alder reaction. Choong Eui Song, Jung Hoon Choi and their groups at Korea Institute of Science and Technology and Hangyang University, Seoul, South Korea, have found that the use of scandium triflate in very small amounts in ionic liquids allows the Diels–Alder reaction to be performed much more rapidly than in many organic solvents, with quantitative yields (Chem. Commun, 2001, 1122). The endo : exo ratio is also excellent at 99 : 1. An additional bonus is that the catalyst can be recovered easily and reused several times by simple phase separation. No loss in activity or yield was noted after 11 cycles. | ||
| Scheme 13 | ||
Fluorous biphase catalysts
Fluorous biphase catalysis is another innovative way to effect reactions and utilise phase behaviour to isolate reaction components, this time by the temperature-dependent miscibility of certain perfluorinated solvents and hydrocarbon solvents. One of the difficulties associated with this technique is the need to functionalise catalysts with fluorous ‘pony-tails’ to solubilise them in the reaction medium, which is typically much less polar than even a hydrocarbon. Shannon Vinson and Michel Gagné of the University on North Carolina, USA, have now demonstrated that a polymer- supported hydrogenation catalyst (based on a Rh-diphosphine copolymerised with ethylene glycol dimethacrylate) works extremely well under fluorous biphasic conditions (Chem. Commun., 2001, 1130). They found that hydrogenation of methyl cinnamate was complete 2–3 times faster than in conventional solvent systems. Surprisingly, they also observed that reaction rates increased with increasing perfluoro content of the solvent mixture. While separation of a heterogeneous catalyst does not require a biphasic solvent system, the observation that there may be potential benefits of using fluorous solvent systems (and possibly much more so for oxygen- based oxidations) is an importantobservation. | ||
| Scheme 14 | ||
European teaching on renewable resources
In the framework of a European Curriculum Development Programme, a group of European universities are collaborating in order to create a European masters programme on renewable resources. The CDA (Curriculum Development Advanced) programme aims at starting the master studies in 2004, if approved by the EU at that time
In response to the trend to promote and utilise more renewable resources based products in industry, the need to translate this trend in education became urgent. Further, the need to train people in an European environment in order to prepare them for the challenges of global thinking and acting, supported the idea to create a think tank for the organisation of a European masters programme. Several European universities active in the domain of renewable resources became involved in the CDA programme and are developing a practical system for the masters programme. Among the universities involved are:• Akademia Rolnizca (Wroclaw, Poland)
• Institut National Polytechnique de Toulouse (Toulouse, France)
• Joensuun Yliopisto (Joensuu, Finland)
• Justus-Liebig-Universität Giessen (Giessen, Germany)
• Kauno Technologijos Universitetas (Kaunas, Lithuania)
• Sveriges Lantbruksuniversitet (Uppsala, Sweden)
• Universität für Bodenkultur Wien (Vienna, Austria)
• University of York (York, UK)
• Westfälische Wilhelms-Universität Münster (Münster, Germany) and
• Ghent University (coordinator, Ghent, Belgium).
The aim of the programme is to educate young academics with the ideas of
the use of renewable resources, sustainable development and green
chemistry. Within the programme, special attention will be paid to topics
related to:
• European policy and socio-economical aspects of the use
of renewable resources
• the production of renewable resources
• downstream processing
• non-food applications of saccharides, proteins, oils and
fats
• renewable energy
• green chemistry
• wood and fibres
• high-value added materials etc.
On top of the technical and technological aspects of the programme the students will benefit from the international environment and the experience offered by the master programme since the courses of this two-year programme are planned to be given in a modular system in at least three (or four) different universities. This means that students will need to move to different countries, thus accumulating major cultural, lingual and international experience which will be of major importance for a job career in a unified European market.
The cost of the master programme is planned to be supported strongly by the European Community and the student and staff mobility programmes of the EU. In this way students will be in touch with European experts of the different topics included in the programme. Within the programme, much attention will also be paid to practical work and case studies, as well as to a research period in a university of choice within the programme. Flexibility and tailor made programmes for students with a certain background will be a major concern of the organisation. Therefore, the programme will be suitable for agricultural engineers, chemists, foresters, biochemists, bio-engineers etc. In view of the development of the educational system within Europe, the programme might become a preferred education option in a 3 + 2 education system as proposed in the Bologna and Prague declarations of the EU.
Universities and institutes interested in the further development and organisation of this master programme on renewable resources may contact: Prof. Dr. ir. Christian Stevens, Department of Organic Chemistry, Faculty of Agricultural and Applied Biological Sciences, Ghent University, Coupure links 653, B-9000 Gent, Belgium, e-mail: Chris.Stevens@rug.ac.be; Fax : 0032 (0)9 264 62 43.
Towards a metabolic society: a thermodynamic view
Jakob de Swaan Arons and Hedzer J. van der Kooi of the Laboratory of Applied Thermodynamics and Phase Equilibria, at Delft University of Technology in The Netherlands† present a thermodynamic argument that our society should develop to become a metabolic society,1,2 involving more efficient conversion and disposal of energy and material. Most living systems fulfill the requirements of a metabolic society, directly (plants) or indirectly (animals).3 The notorious exception is man as from the moment that he got involved in what is called ‘the industrial society’. How can this unfortunate exception be repaired?
Introduction
The 20th century showed the full development of an industrial society characterized by mass production, mass consumption and mass waste disposal.1 For many countries this has produced prosperity and an unprecedented economic growth with, in many respects, an improved quality of life. But gradually less attractive consequences of this development became apparent, like the dependency on fossil fuels and the impact on the environment that seem to have taken global proportions. So on the way to the 21st century we began to realize that this posed us for a Trilemma, where we are challenged to devise a way to harmonize economic growth, resource supply and preservation of the environment.4Prominent representatives of industry, government and academia, recognizing this Trilemma, therefore have called for ‘sustainable development’, often with a less precise description albeit with the best of intentions. Sawa for example proposes that the 21st century should see the development of a post-industrial society, the metabolic society, characterized by viable consumption levels, limited waste, active recycling, energy savings and longer product lives.1 This is a very interesting proposal and we felt the need to try and identify the characteristics of such a society. Therefore we have turned to living systems and identified some of their basic requirements with regard to the conversion of energy and matter.3 Next we tried to analyze the way we ‘manage our house’ by starting from a simple economic picture and by extending it to what we believe is a picture of a self-sustaining, rather than of a self-exhausting society. Finally we present a perspective of the global industrial society from how it is to how it should develop.
The metabolic society
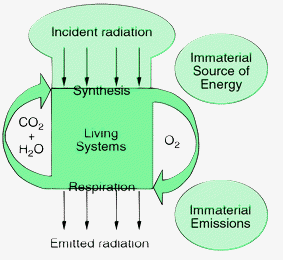
When Sawa launched the term ‘metabolic society’ he may have had in mind a society that lives in dynamic equilibrium with its environment, producing enough food and materials to sustain its passive and active life and absorbing in a natural way all its waste produced, like a cow living on a pasture of sufficient area, the total system powered by the sun. This is what many believe to be a sustainable society. Angela Merkel, physicist and former German Minister of the Environment defines sustainable development as ‘Using resources no faster than they can regenerate themselves and releasing pollutants to no greater extent than natural resources can assimilate them’.5 This was of course the situation before the industrial revolution and man fitted quite nicely in such a society. But then he got engaged in the industrial society, and by doing so stepped out of the metabolic society. A point was reached that ‘nature could not keep up with us any longer’, as Yoda puts it.4Morowitz has summarized some characteristics of living systems.3 They prevail in a permanent non-equilibrium with respect to their environment. This state is sustained by a permanent energy source. They display structure and prevail in an active or passive mode. The energy source needs a sink for disposal of its degraded form, while stripping energy from its useful part requires at least one material cycle. Fig. 1 shows schematically the metabolic society. Radiation from the sun is stripped from its useful component and transformed into mechanical, electrical or chemical energy. Overall, solar radiation is converted into the same amount of useless heat to the environment while sustaining a structured, non-equilibrium system of plants and animals, requiring at least one material cycle of synthesis and respiration involving CO2, H2O and O2. In this cycle the smallest living systems, the microbes, are most numerous and essential. They keep matter in cycles and are also responsible for the final degradation step to CO2 and H2O, thus assuring ‘waste’ disposal.
 | ||
| Fig. 1 | ||
The industrial society
Ecology is the study and economy in the management of ‘the house’. So let us see how we manage the house in which we live, the industrial society. Fig. 2 and Fig. 3a picture the classical way of thinking in economics in which capital, production and consumption stand out. However environmental economists pointed to what is missing in this picture: the environment. In thermodynamics we know all too well that we should always look at the system and its environment. Aware of this, environmental economists emphasize the importance of the interaction with the environment via extraction and emission (Fig. 3b). This picture expresses somehow the less favourable aspects of an industrial society. Ishikawa condemns this by stating that ‘we contaminate the environment by squandering natural resources’.6 In this respect the industrial society is markedly different from the metabolic society and much more primitive. At one time man stepped out of nature′s cycles. We may now have reached a state in which we are called back to order. Fig. 3 also allows us to point to what it takes to make the transition back from industrial to metabolic. | ||
| Fig. 2 | ||
 | ||
| Fig. 3 | ||
We realized that Fig. 3b can be further extended by closing the cycle
between emissions and extraction. The amount of work that this requires
necessarily has to be paid for by a renewable source of work, Fig. 3c.
Otherwise other non-closed cycles would be introduced. This picture helped
us to arrive at a possible thermodynamic definition of
sustainability:
• close all product cycles
• drive all cycles with renewable energy
• do it efficiently
Thermodynamic parameters can now be defined7 to calculate and measure to what extent a society is sustainable. It may not make sense to make use of renewable energy while this is done inefficiently, nor might it suffice to use renewable energy efficiently when material cycles are not closed. By the way, an interesting consequence of this analysis is that the generating cost of a fossil fuel must always be higher than that of a corresponding amount of a renewable energy source.
Thermodynamic perspective
Thermodynamics has provided us with two very useful concepts, both originating in the two fundamental laws: available work and lost work.8 Both concepts can be calculated and easily communicated to non-experts as we hope to demonstrate.Fig. 4 pictures the fate of work, as available in ‘methane’ (natural gas), in the chain from gas production to utilization. The picture clearly shows the transformations that take place in time and allows some important observations. First and foremost it visualizes the first and second law. Although energy is conserved, its quality, i.e. its available work content, diminishes and finally disappears while the amount of lost work increases in time. Secondly it makes us aware that a material resource of energy has depleted and that its transformation is accompanied by emissions due to the law of mass conservation: mass stored underground and mass extracted from the atmosphere are transformed and sent into the atmosphere. Thirdly the application, electricity consumption, does not need a material source of energy. An immaterial source of energy as solar radiation can also provide electricity. Finally, if we make a life cycle analysis of this process, we notice that the process is not a closed cycle.
 | ||
| Fig. 4 | ||
Fig. 5 tries to picture that the work originally available in fossil fuel is used to pay for global energy needs, is lost in time and that the mass in which this work was stored is conserved and emitted. The overall conclusion is that as most (>90%) of the fossil fuel is used for ‘energy’ purposes, i.e. not requiring a material resource for the work required, a massive flow of matter is generated, unnecessarily.
 | ||
| Fig. 5 | ||
At the same time we should realize that the work available in the incident solar radiation is orders of magnitude greater than what the world in its human activities transforms into lost work.2 The fate of this available work however is the same as that in most of the consumed fossil fuel: lost work. The work is dissipated and although the radiation from the earth is still valuable for bodies at a lower temperature than the earth, it has lost its power for the benefit of the earth (Fig. 6).
 | ||
| Fig. 6 | ||
The challenge is now to tap the work available in the incident radiation and thereby to eliminate largely the massive material emissions associated with the consumption of fossil fuel: the dematerialization of energy sources. Fig. 7 tries to picture this. In contrast to Fig. 5 the required work is now available in an immaterial resource and consequently no net flow of matter is associated with it: ‘the solar fueled globe’ where the dissipation of the work available in solar radiation (Fig. 6), is now delayed for the benefit of the globe′s industrial activities. However, here is a point of concern. There is strong evidence2 that the globe′s ecological capacity is no longer sufficient to fulfill our energy needs by photosynthesis. This then asks for alternative extraction of the work available in solar radiation and, possibly, for a further reduction in human material needs.
 | ||
| Fig. 7 | ||
Conclusion
In comparing characteristics of a metabolic society with those of an industrial society one cannot help but conclude that the latter is far more primitive in terms of access to, and conversion and disposal of energy and matter. Man can learn much from the study of natural processes and may have to conclude that he should become part again of natural cycles much as he did before the industrial revolution. This may imply a simultaneous reduction of his material needs.Acknowledgement
We express our appreciation to economists/physicists Professor Robert Ayres (INSEAD, France) and Dr. Stefan Baumgärtner (University of Heidelberg, Germany) for all we learned from them at the Gordon Research Conference on Thermodynamics, Pisa, Italy, April 1999.References
- T. Sawa (ed.). Creating an Affluent Society. In Harmony with the Earth. Proposals for Japan, CRIEPI, Tokyo, Japan, 1996. Search PubMed.
- M. Wackernagel and W. Rees. Our Ecological Footprint. Reducing Human Impact on the Earth, The New Catalyst, 1996. Search PubMed.
- H. J. Morowitz. Energy Flow in Biology, Oxbow Press, 1979. Search PubMed.
- Yoda (Ed.). Trilemma. Three Major Problems threatening World Survival. Central Research Institute of Electric Power Industry, Tokyo, Japan, 1995. Search PubMed.
- A. Merkel. The Role of Science in Sustainable Development, Science, 281, 17 July 1998. Search PubMed.
- E. Ishikawa. Energy Recycling in the Edo Period. Editorial in the Asahi Shimbun, Dec. 17, 1996. Search PubMed.
- J. Dewu1f, J. M. Mulder, M. M. D. Van den Berg, H. Van Langenhove, H. J. Van der Kooi and J. De Swaan, Arons, J. Illustrations Towards Quantifying the Sustainability of Technology. Green Chem, 2000, 2, 108–114 Search PubMed.
- J. D. Seader, Thermodynamic Analysis of Chemical Processes, MIT Press, 1982. Search PubMed.
A greener future with biodiesel
Jeffrey Hardy, a Green Chemistry Teaching Associate at the Clean Technology Centre at the University of York, UK, describes the sources and production of this renewable transport fuel, and argues the case for its wider acceptance
UK case studyAlthough significant progress regarding the introduction of biodiesel has been made in several European countries in the UK there still remain several hurdles to overcome. The major obstacles are clearly outlined by examining the case of Martin Steele.10,11 Using his ingenuity Mr. Steele has developed a transesterification process to turn used chip pan fat into perfectly functional biodiesel. In fact he has successfully run his Volvo car on his biodiesel fuel (produced in his Manchester back garden) for over 18,000 miles. The car engine was examined after 15,000 miles and was found to be virtually corrosion and carbon deposit free. This proves the validity of the fuel, and at a small scale production cost of 12p per litre it would appear to be competitive with regular diesel. The major problem arises when Customs and Excise demand he pays around 50p per litre in road fuel tax. Mr. Steele comments on this by saying ‘The Government talks about saving the environment and cutting down on emissions, buttaxes biodiesel at the highest rate in the world. It’s daft!’ He also claims that the problem arises from a ‘wrong calculation’ by MAFF in a report to the government back in 1996 ‘One wrong figure in the report has set biodiesel production in the UK back by 5 years’. Furthermore, Mr. Steele attacks the UK Government on the subject of road tax ‘The Chancellor last year took £55 off road tax on small cars with no measurable benefit in terms of greenhouse gas savings. That cost the Exchequer £110 million. For the same amount of cash, we could have a brand new biodiesel industry in the UK, creating 2000 new jobs. It would have supported the production of 500 million litres of biodiesel, saving in round terms, half a million tonnes of carbon emissions’.Mr. Steele continues his campaign to have the duty on biodiesel reduced. He is also involved in trying to set a larger scale production plant in collaboration with other biodiesel enthusiasts. |
Introduction
Biodiesel is a renewable transport fuel made mainly from vegetable oils. It is not a new fuel; in fact Rudolf Diesel ran his original engine on peanut oil in 1900. The reason why people are not familiar with biodiesel is that because mineral oil derived diesel has been cheaper and more available for the majority of this century. The oil crisis in 1973 reawakened interest in biofuels when mineral oil prices skyrocketed. Now, even though the oil prices have fallen again the interest in biofuels has not diminished due to the environmental advantages of biodiesel.Biodiesel is quite simply a fuel made from vegetable oil. More precisely it is defined as the mono alkyl esters of long chain fatty acids derived from renewable lipid sources. The processing required to produce biodiesel is minimal, firstly oil has to be extracted from the crop and secondly a very simple chemical modification of the oil has to be carried out. Biodiesel can be used in an unmodified diesel engine as either pure biodiesel or as a mixture of biodiesel and conventional mineral oil based diesel. A tractor fuelled by biodiesel derived from sunflower oil has been successfully driven from Lands End to John o’ Groats (Fig. 1).
 | ||
| Fig. 8 Tractor fuelled by biodiesel.1 | ||
The source of oil for biodiesel production depends on the agriculture of the country where it is produced. For instance in Europe it is produced from oilseed rape and sunflower oil, in North America from soya oil and in South East Asia from palm oil.
There are several key advantages biodiesel possesses over conventional diesel. Perhaps first and foremost is the fact that biodiesel is neutral with regard to carbon dioxide (CO2) emissions and consequently it does not add to global warming. This is in stark contrast to conventional diesel, which produces three tonnes of CO2 for every tonne of diesel burnt. Biodiesel is also non-toxic (less toxic than table salt according to the EPA) and degrades quickly in the environment leaving innocuous products. Unlike mineral oil diesel, biodiesel has a negligible sulfur content and consequently it does not add to acid rain. Biodiesel is a much better lubricant than conventional diesel fuel and extends engine life—a German truck won an entry in the Guinness Book of Records by travelling more than 1.25 million km (780,000 miles) on biodiesel with its original engine.2 Biodiesel is also the only alternative fuel to have passed the stringent health effects testing requirements of the US Clean Air Acts Amendments of 1990.3
Methods of producing biodiese
Vegetable oils can be used directly as diesel fuels, but the inherent viscosity of the oils is often a problem. This can be solved by the formation of micro-emulsions with solvents such as methanol and ethanol. However, neat vegetable oils and the emulsions both cause coking and valve blocking in engines due to problems deriving from oil deterioration and incomplete combustion.The most widespread method for production of biodiesel is through a transesterification process. Natural fats and oils exist as glycerides, a triester of fatty acid and glycerol, which are naturally viscous by nature. The transesterification process involves the reaction of a fat or oil with an alcohol to form esters and glycerol (both of which are commercially saleable products).4 A general reaction is shown below in Scheme 1.5
 | ||
| Scheme 15 Transesterification of triglycerides with alcohol. | ||
A catalyst is generally used to improve the reaction rate and yield. Because the reaction is reversible, excess alcohol is used to shift the equilibrium to the product side (the alcohol is recycled). Methanol is the most commonly used alcohol because of its low cost and physical and chemical advantages (polar, shortest chain alcohol and sodium hydroxide, a basic catalyst, is soluble in it).
The ratio of alcohol to glyceride required for reaction is dependent on whether the reaction is catalysed by acid or base. An acid catalysed reaction needs a ratio of 30∶1, whereas a base catalysed reaction (ca. 1% w/w for both acid and base) requires a ratio of 6∶1. A major point is that the base catalysed system is intolerant to water content in the vegetable oil, whereas acid catalysis can tolerate water. Enzyme catalysts have also been used.6
A typical continuous reactor used in the production of biodiesel from various sources is shown below in Fig. 2. The oil is added separately to the alcohol and catalyst. The reagents are passed through a heated column where reaction takes place. The esters are then separated from glycerine by gravity.
 | ||
| Fig. 9 A continuous transesterification reactor.7 | ||
Other countries producing biodiesel
Biodiesel is seen as an important tool for the EU to meet its emission reduction target (Kyoto agreement). Specific legislation to promote and regulate the use of biodiesel is in force in various countries (including Austria, France, Germany, Italy and Sweden). Europe currently produces 700,000 tonnes/year of biodiesel and has set production targets of 2.3 Mtonnes by 2003 and 8.3 Mtonnes by 2010 to reduce greenhouse and air polluting gas emissions.8 Germany is on course to produce 1 Mtonnes/year by 2003 thanks to a zero duty rate on the fuel. There are several excellent examples of European countries increasing their usage of biodiesel:• Germany has been producing biodiesel since 1992, does not tax the fuel and has plans for an annual production of 400,000 tonnes. There are over 700 filling stations in Germany selling biodiesel.
• Many French towns have buses running on a mixture of biodiesel and fossil diesel. It is distributed, amongst others by Elf and Total. In fact most fossil fuel diesel in France is mixed with biodiesel today.
• In Sweden it is sold in a mixture with fossil diesel. Also in Stockholm they have greatly cut pollution by using ethanol as a fuel additive.
• Public transport in Rotterdam, Copenhagen, Olso, Zurich, Luxembourg, Lisbon and Barcelona has used biodiesel with excellent results.
Biodiesel in the UK
Road transport is now the UK’s third largest source of CO2 emissions. The Governments Climate Change Programme sets out an ambitious package of measures across all sectors, which is expected to reduce greenhouse gas emissions by 23% by 2010 relative to 1990. This will include a substantial reduction in the use of carbon rich fuels in the transport sector. In the UK over 500,000 ha of land are set aside yearly; it is feasible that much of this land could be used for oilseed rape production. Surprisingly, much of the oilseed rape production in the UK is actually used in other European states.Biodiesel has been tested by several organisations in the UK, all of which demonstrated the suitability of biodiesel for general use. In the most recent budget the Chancellor of the Exchequer, Gordon Brown, announced that biodiesel would qualify for a 20p per litre tax rebate. This will mean a tax on biodiesel of about 25p per litre to come into effect after approximately one year. Although this was widely considered a step in the right direction pressure groups have indicated that they would prefer the duty on biodiesel to be more like 10p per litre (in some EU countries there is no duty on biodiesel). With a reasonable duty on biodiesel it is predicted that farming industry could provide between 5 and 10% of the UK diesel needs over the coming years.9
The future
The UK is perceived within the EU as dragging its heels behind the biodiesel programmes in other member states. However slowly, it seems that the political mood is changing, with recent announcements of the lowering of the fuel duty on biodiesel and other more positive signs regarding future use of biofuels.The amount of farmland set aside yearly could be used to grow crops such as oilseed rape. Through calculation it has been estimated that up to 10% of the UK’s diesel consumption could be accounted for by biodiesel derived from homegrown crops, which is a significant amount in terms of saving fossil fuels and CO2 emissions.
References
- http://www.newtonrigg.ac.uk/agric_ forestry/millennium.html.
- http://journeytoforever.org/biodiesel.html.
- http://www.biofuels.fsnet.co.uk/.
- D. S. Bradin, US patent 5,578,090, Biodiesel fuel..
- http://www.newtonrigg.ac.uk/Agric_ Forestry/Chemistry.htm.
- K. Ban, M. Kaieda, T. Matsumoto, A. Kondo and H. Fukuda, Biochem. Eng. J., 2001, 8, 39 CrossRef CAS.
- Picture adapted from W. R. Trent, US Patent 2, ( 1945), 383–632..
- http://www.biodiesel.co.uk/press_ release/labour_applies_brakes.htm.
- http://www.biodiesel.co.uk/press_ release/biodiesel_gets_a_start.htm.
- R. Cave, The Big Issue in the North, 2001, 366, 5 Search PubMed.
- Mendick and J. Champkin, The Independent, 22nd October 2000. Search PubMed.
Chemical companies take energy management to heart
This article from the Energy Efficiency Best Practice Programme discusses the importance of chemical companies to take energy management to heart
GETTING STARTEDGPG 200 A Strategic Approach to Energy & Environmental ManagementGPG 190 Energy Efficiency Action PackVideo VI 014 The Bottom LineInteractive software The Energy ManageMent Advisor - EMMA″ Focus - The Manager′s Guide to Reducing Energy BillsACBE 001 Energy Saving Guide for Small Businesses Institute of Director′s Making Savings by Managing Energy - a Director′s Pocket BookPLANNING & ORGANISINGGPG 119 Organising Energy Management - Corporate Approach GPG 186 Developing an Effective Energy PolicyGPG 167 Organisational Aspects of Energy ManagementGPG 200 Assessing Energy ManagementGPG 119 Energy Management Matrix |
Introduction
The advent of the climate change levy (CCL) in April 2001 has provided the impetus for many chemical companies to look again at how much energy is used on site. The chemical sector has now finalised the Negotiated Agreement with the Government and this means that chemical companies will benefit from an 80% discount on the CCL. However, to retain this discount in future years, chemical companies must make the agreed energy savings.As many chemical manufacturing organisations are re-launching their energy management strategies, there is an increasing requirement for support and guidance. Managers must use both time and financial resources as effectively as possible and energy management should now be part of any good management system. Energy and utility costs are frequently viewed as an ‘overhead’ but they are actually a controllable cost. As with any commodity, energy cannot be managed effectively unless it can be closely measured. It is not enough to just have a meter at the site boundary. Fortunately larger companies, with sites that have several plants and other buildings, have extra meters for cost allocation purposes. This data can be used as a key part of the energy management process.
Energy management is also not just about one person doing all the work. It is important to educate the whole workforce, starting at management level. Information is the key. Making sure everyone gets the message will make the energy manager’s job a lot easier. There is a host of information available within the Government’s Energy Efficiency Best Practice Programme (EEBPP), to help energy managers make the best use of energy inputs. The EEBPP is pioneering a new scheme that will provide specific support to larger chemical manufacturing sites. This takes the form of ‘Partnerships’ between the site and EEBPP, who provide energy audits, training seminars, literature and technical help. The partnership approach is based on discussions between the site and specialists from the EEBPP who ensure that the events are ‘tailor made’ to suit the site’s specific requirements. This advice, when combined with the company’s own expertise, turns potential energy savings into improved profits as well as reducing environmental impact.
Solutia takes free advice
Solutia UK Ltd. operates a chemical manufacturing plant at Newport, South Wales, with seven production plants using organic and/or inorganic processes. Techniques used at the plant include pyrolysis, esterfication and hydrogenation, and the plant operates on a 24-hour shift pattern. The company produces several chemicals such as organic phosphonates, rubber chemicals, phosphate esters and a plasticiser used as the safety interlayer in car windscreens. Solutia recognised that there was plenty of scope for energy reductions at its Newport site. So, the company called on the EEBPP to help them form an energy management team and disseminate the necessary information factory-wide. | ||
| Fig. 10 Solutia, a chemical company working for energy efficiency. | ||
David Lloyd, an EEBPP energy consultant worked with Solutia and created a tailor made programme of talks and discussions. ‘Since the seminar in December, with full attendance of a wide cross section of personnel, we have initiated an Energy Steering Group. The first meeting has been held and over 180 items have been prioritised for investigation or action,’ Keith Agnew, who heads the group, explained. He is encouraged to have such a wide cross- section of support.
The Steering Group has drawn up a long-term plan and set a timescale for actions. ‘Following the seminar, I have ensured that energy efficiency is an area that appears in each line production manager’s goals,’ said Keith Agnew. ‘This is not a one-off event, though, we must deliver energy reductions year-on-year, and make sure we have continuous sustainable improvement’. Keith Agnew is encouraged that managers are so willing to take the energy efficiency message on board. ‘Solutia greatly values the EEBPP’s support and guidance, especially after the success of the seminar last December,’ he said.
Solutia created an inventory and database of the 506 motors in use on site, and defined a motors strategy. As a result, the company will either rewind or replace motors at the appropriate time. If motors are over sized, they will be replaced with a smaller motor. Process optimisation is also an important factor and some improvements were identified. However, as major changes were made a few years ago, the potential for further savings is not as great as in other areas, such as reducing the amount of compressed air used on site.
Solutia uses a large amount of compressed air and the Energy Steering Group carried out a basic leak survey at the end of 2000. The leak rate for the site was considered to be the industry average, and the industry average is high. This means that any energy used to produce leaked compressed air is wasted. ‘To date £60,000 per year of leaks have been identified and the majority have been fixed, with the remainder in a plan to address them when there is an opportunity,’ said Keith. ‘It is important to keep up general good housekeeping measures of tightening valves and turning the air supply off when not in use. We must be able to sustain this improvement into the future,’ he said.
The benefits of having a company-wide Energy Steering Group cannot be over-emphasised. Having managers from all areas gives a true site perspective. Savings that can be made in one place may have cost implications elsewhere. It is important to be able to consider all these aspects together and to have everyone involved. Having gained the commitment of the management team, Keith is now very keen to get the message across to all site staff. ‘This year I will be creating a special topic for line manager meetings that will raise awareness and the importance to us all of energy efficiency. Ultimately, I would like to think we could have 250 energy managers here at Solutia,’ he said.
The EEBPP is able to help chemical companies set up an energy management strategy and support managers in implementing it. Free site-specific advice is provided through dedicated energy efficiency advisers. Several free publications are also available through the Environment and Energy Helpline Tel: 0800 585794, and the most relevant are listed in the box. Alternatively, visit the EEBPP website http://www.energy- efficiency.gov.uk for more details of the services offered.
For more information please contact: Kathryn Stubbing, IMS Public Relations (Tel. 01672 520788; Fax 01672 520789) or Elena Stewart, ETSU (Tel. 01235 433967; Fax 01235 433906).
Footnote |
| † Delft University of Technology, Julianalaan 136, 2628 BL Delft, The Netherlands; email: a.m.c.barrow@tnw.tudelft.nl |
| This journal is © The Royal Society of Chemistry 2001 |
