Characterisation of mineralogical forms of barium and trace heavy metal impurities in commercial barytes by EPMA, XRD and ICP-MS
Tariq M. Ansari, Iain L. Marr and Alison M. Coats
Department of Chemistry, University
of Aberdeen, Meston Walk, Old Aberdeen, UK AB24 3UE
First published on 22nd November 2000
Abstract
This study was carried out to characterise the mineralogical forms of barium and the trace heavy metal impurities in commercial barytes of different origins using electron probe microanalysis (EPMA), X-ray diffraction (XRD) and inductively coupled plasma mass spectrometry (ICP-MS). Qualitative EPMA results show the presence of typically eight different minerals in commercial barytes including barite (BaSO4), barium feldspar, galena (PbS), pyrite (FeS2), sphalerite (ZnS), quartz (SiO2), and silicates, etc. Quantitative EPMA confirms that the barite crystals in the barytes contain some strontium and a little calcium, whereas trace heavy metals occur in the associated minerals. Analysis of aqua regia extracts of barytes samples by ICP-MS has shown the presence of a large number of elements in the associated minerals. Arsenic, copper and zinc concentrations correlate closely in all 10 samples. The findings suggest that barytes is not, as traditionally thought, an inert mineral, but is a potentially toxic substance due to its associated heavy metal impurities, which can be determined by an aqua regia digest without the need for complete dissolution of the barite itself. X-ray powder diffraction was not informative as the complex barite pattern masks the very weak lines from the small amounts of associated minerals.
Introduction
Barytes (the naturally occurring rock form of BaSO4) is the standard densification agent used in drilling fluids world-wide. Its high specific gravity (4.5 g cm−3 for barium sulfate), low Moh's hardness (3.0–3.5), low seawater solubility, low abrasive properties, chemical inertness and easy handling are the main characteristics that make it suitable for increasing the density of drilling fluids to control the hydrostatic pressure.1 It is abundant and commercial deposits are sufficiently widespread to make it deliverable to well sites at an acceptable cost. It is estimated that total world mine production of barytes was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes
in 1998. In USA, about 3
000 tonnes
in 1998. In USA, about 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of ground barytes
from both domestic production and imports were sold in 1998, nearly 90%
of which was used as a weighting agent in oil and gas drilling fluids: a typical
consumption would be 1500 tonnes per well, 90% of which is discharged
to the marine environment after use.2,3
000 tonnes of ground barytes
from both domestic production and imports were sold in 1998, nearly 90%
of which was used as a weighting agent in oil and gas drilling fluids: a typical
consumption would be 1500 tonnes per well, 90% of which is discharged
to the marine environment after use.2,3The occurrence of mineral impurities in barytes has been cited in the literature.4–7 These may include alkali metals, alkaline-earth metals, heavy metals and rare earth elements, depending on the source of the barytes. These elements occur either in small inclusions of the other minerals or as ionic substitutions in the crystal lattice. The most common associated minerals are anhydrite, calcite, fluorite, quartz, celestite, alumina, dolomite, galena, sphalerite, pyrite, manganite, stibnite and siderite.
Concern has been expressed by some regulatory authorities, i.e., the Paris Commission (PARCOM) and the United States Environmental Protection Agency (US EPA) controlling offshore drilling operations regarding the potential for bioaccumulation in marine animals of the trace heavy metals present in drilling muds discharged to the marine environment and the final destination of these metals in man. In the North Sea area, discharges are monitored and controlled through PARCOM which has classified mercury and cadmium and their compounds along with organo-halogens and petrogenic hydrocarbons as substances that are persistent, toxic and have noxious properties. A further six elements—arsenic, chromium, copper, lead, zinc, nickel—are included in a further list of ‘less noxious elements’. PARCOM has suggested a strict control of discharge of these pollutants to the North Sea.8 This calls for reliable and cost-effective analytical methods: the US EPA, for example, stipulates that barytes for such applications should not have more than 1 mg kg−1 of mercury or 3 mg kg−1 of cadmium.9
Because of the general perception of barytes as an inert chemical, environmental impacts of such potential toxic heavy metals have been rather played down by the oil industry and the scientific community in the past. With growing environmental awareness and more stringent legislation, the oil industry should be reconsidering this view and collecting information on the bio-availability of trace heavy metals in barytes. The mode of occurrence of heavy metals in commercial barytes is thus an important aspect from both an analytical and an environmental point of view. The current investigation was planned to reveal the chemical nature and ‘availability’ of heavy metals present in barytes, so that appropriate analytical procedures could be selected for their determination, and their potential to cause an environmental hazard could be better assessed.
The aims of this study were, therefore, to: (a) identify the mineral forms of barium and of the trace heavy metal impurities in commercial barytes; (b) to find out which trace heavy metals were present within the barite matrix and which as associated minerals; (c) to quantify the relative proportions of barium as barite and as other barium-containing minerals present in barytes.
Experimental
The background to the barytes
Barytes of different origin were collected from commercial suppliers. Details of apparent colour, origin and suppliers of the barytes are listed in Table 1. All materials were obtained as fine powders having particle sizes in the range 1–25 µm. The materials were heated in an oven at 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 1 h to remove
any moisture and then stored in a desiccator prior to carrying out further
investigations.
°C for 1 h to remove
any moisture and then stored in a desiccator prior to carrying out further
investigations.
| Barytes | Colour | Origin/supplier |
|---|---|---|
| B1 | Grey | China, Anchor Drilling Fluids |
| B2 | Grey | Scottish ore |
| B3 | Light brown | North Africa |
| B4 | Bluish grey | India |
| B5 | Reddish brown | USA, Bariod Drilling Fluids Inc., USA |
| B6 | Light brown | Barytes, B6, B7, B8, B9 and B10 were collected from different locations of the world, supplied by Schlumberger Cambridge Research, Cambridge, UK |
| B7 | Grey | |
| B8 | Light brown | |
| B9 | Grey | |
| B10 | Greyish purple |
Equipment
Cameca SX51 EPMA (Cameca, France) equipped with four wavelength dispersive spectrometers (using lithium fluoride (LiF), pentaerythritol (PET), thallium acid phthalate (TAP) and pseudo-crystal (PC1) analysing crystals). The instrument was operated using Cameca software in a Sun Microsystem environment. Sample preparation used an EMITECH K950 carbon coater (Emitech, England), an Ecomet III Grinder (Buehler Ltd, IL, USA), polishing plates and accessories (Struers, Copenhagen, Denmark). The Stoe Stadi/P automated diffractometer (Stoe, Darmstadt,Germany) was controlled by a DEC MicroVAX computer. A Spectromass 2000 ICP-MS (Spectroanalytical Instruments, Germany). CEM Model MDS-81D (UK) was used to analyse extracts of samplesChemicals
AnalaR grade chemicals supplied by BDH were used throughout this study unless otherwise mentioned. Isopropanol, acetone, BaSO4 (reagent grade), SrTiO3, CaSiO3, HCl (Aristar) and HNO3 (Aristar) were also used.Sample preparation and analytical measurements
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
for 2 h to cure. The resin blocks were polished in four stages using: (i)
metallographic grinding paper (GRIT P600); (ii) metallographic
grinding paper (GRIT P1200); (iii) a 6 µm
plate with a diamond polishing paste and paraffin oil; and (iv)
a ¼ µm polishing plate with 0.3 µm alumina
powder and paraffin oil. To avoid any contamination from the coarse grit material
imported from the previous polishing stage, the samples were washed thoroughly
with isopropanol in an ultrasonic bath after each stage. Finally, samples
were coated with carbon (approx. thickness ∼10–20 nm).
EPMA measurements comprised qualitative, quantitative and image analysis.
°C
for 2 h to cure. The resin blocks were polished in four stages using: (i)
metallographic grinding paper (GRIT P600); (ii) metallographic
grinding paper (GRIT P1200); (iii) a 6 µm
plate with a diamond polishing paste and paraffin oil; and (iv)
a ¼ µm polishing plate with 0.3 µm alumina
powder and paraffin oil. To avoid any contamination from the coarse grit material
imported from the previous polishing stage, the samples were washed thoroughly
with isopropanol in an ultrasonic bath after each stage. Finally, samples
were coated with carbon (approx. thickness ∼10–20 nm).
EPMA measurements comprised qualitative, quantitative and image analysis.Qualitative measurements. Examination of the backscattered electron images (BSE) of the barytes samples indicated the presence of several different phases. These phases were differentiated by eye according to grey shades. Each phase was selected in turn and from the wavelength spectrum its elemental composition was identified. In this work, an acceleration voltage of 20 kV and a beam current of 30–60 nA was used.
Quantitative measurements. Grains of the mineral barite were selected for quantitative analysis with the help of BSE images and 20 points per sample were analysed. An accelerating voltage of 20 kV and a 40 nA beam current were used. The lines, Sr Lα , Ba Lα , Ca Kα and S Kα (the elements shown to be present in significant quantities) were measured. A counting time of 20 s per point was used. Counts for each element were corrected for: (1) absorption of X-rays, (2) backscattering of electrons and (3) fluorescence. BaSO4, SrTiO3 and CaSiO3 were used as standards for Ba(II), S(VI), Sr(II) and Ca(II). The results from the quantitative analysis were calculated as wt.%, as atom% and finally as stoichiometric ratios. Oxygen was calculated by stoichiometry.
Image analysis. Element maps of Ba, Si and S from a representative area of the barytes samples were recorded to determine proportions of barium in barite and in other minerals. An accelerating voltage of 20 kV and a 40 nA beam current was used. Area calculations were made for total Ba in the image frame, for barium associated with Si, and for barium not associated with sulfur.
Results and discussion
Visual examination
On a macroscopic scale, the distinguishing feature of the barytes samples was the colour, from pale grey (<2% iron) through light brown (ca. 3% iron) to red brown (>4% iron).Traditionally, one would examine rock samples by transmission optical microscopy of a thin section, to identify the minerals present. This approach is not appropriate under the present circumstances as the samples are finely powdered and could not therefore be ground down to a standard thickness of the thin section. EMPA, on the other hand, offers many advantages over optical microscopy, above all the capability to give elemental analysis of individual particles.
Qualitative EPMA of mineral components of barytes
Initially, back-scattered electron images were collected of the powdered barytes samples, which showed typically four or five clearly different shades of grey. This served to identify phases with different mean atomic number. Most of these phases appeared to be confined to single grains but frequently clusters were also present which included two, three or even four phases intimately linked. Such clusters are known as lithic grains, i.e., pieces of rocks associated to the barytes when the ore was crushed.X-ray emission spectra of all the mineral phases in the barytes were recorded using the wavelength dispersive spectrometers. For each of the phases identified, their constituent elements and a possible mineral assignment are listed in Table 2, together with the list of samples in which they were found. Only strontium is associated with the barium, all the other metals detected are present in minerals other than the barite.
| Phase | Elemental composition | Probable mineral | Occurring in samples |
|---|---|---|---|
| 1 | Ba (Sr), S, O | Barite | All samples |
| 2 | Ba, O, Si, Al, K | Barium feldspar | 1, 5, 7, 9 |
| 3 | Fe, S | Pyrite | 1, 2, 3, 4, 6, 10 |
| 4 | Pb, S | Galena | 2, 3, 5, 8, 10 |
| 5 | Zn, S | Sphalerite | 1, 2, 3, 6, 10 |
| 6 | Ca, S, O (Fe, Mn) | Anhydrite | 10 |
| 7 | Si, O | Quartz | 2, 4, 6, 7, 8 |
| 8 | Al, Si, O, K | Orthoclase | 3 |
| 9 | Ca, Si, O,K, Mg, Mn, Al, S, Fe | Various silicates | 1, 4, 5, 7, 9 |
Literature on barytes has indicated the presence of associated minerals including quartz, jasperoid, calcite, dolomite, siderite, witherite, rhodochrosite, fluorite and various sulfides especially pyrite, chalcopyrite, sphalerite and galena in the vein and in cavity filling deposits. On this basis, and on the qualitative EPMA results from the present study, the minerals in commercial barytes samples investigated are thought to be barite (BaSO4), galena (PbS), sphalerite (ZnS), barium feldspar, pyrite (FeS2), quartz (SiO2), hematite (Fe2O3) and silicates. It should be added here that EPMA also showed its limitations: due to its high limits of detection, it was unable to detect several other trace metals known to be present, as shown by ICP-MS, including Cu (at ca. 10–30 mg kg−1), Ag, As, Cd, Cr and Hg (at ca. 1–10 mg kg−1) .
Characterisation of minerals in barytes by XRD
XRD can often provide a rapid and accurate identification of the crystalline phases present in a solid material. Fig. 1 shows XRD patterns of the barytes B1, B4, B5 and of a synthetic BaSO4 standard. The patterns of the barytes matched well with that of the standard BaSO4 (24-1035, Powder Diffraction File) with quartz as the additional component. However, the small amounts of the associated minerals could not be identified due to overlapping of the major lines from the barite. We must conclude that, unless a prior physical separation of minor minerals is carried out, XRD is not an appropriate technique for characterising commercial barytes. | ||
| Fig. 1 XRD patterns of barytes, B1, B4, B5 and synthetic BaSO4 standard. | ||
Quantitative EPMA of the mineral barite in barytes
The mineral name barite refers to pure BaSO4. However, the isomorphic replacement of several per cent of the barium in barytes by strontium is not unusual because of the close similarity of the ionic radii of the two elements, 1.35 Å and 1.13 Å, respectively. Although continuous solid solution series from barite to celestine occasionally occur in the natural environment, each mineral is usually found with only a low substitution ratio. Appreciable replacement of Ba by Pb or by Ca is uncommon, although up to 30 mol% PbSO4 in barite has been reported.10 Rarity of natural solid solution between BaSO4 and PbSO4 may be a consequence of the different origins of the two minerals, anglesite (PbSO4) being an oxidation and weathering product of galena.10Table 3 shows the stoichiometric ratios of the major elements detected in the barite phases, calculated against S = 1. That the barite does contain some strontium, and that this element is replacing the barium is seen by the very narrow spread of Ba + Sr mole ratios to sulfur, 0.98 ± 0.01.
| Ba | Sr | Ba + Sr | S | O | |
|---|---|---|---|---|---|
| a Calculations based on mean of 20 points; voltage 20 kV; beam current 40 nA and size 2 µm. | |||||
| B1 | 0.97 | 0.01 | 0.98 | 1.00 | 4.00 |
| B2 | 0.97 | 0.01 | 0.98 | 1.00 | 3.98 |
| B3 | 0.95 | 0.02 | 0.97 | 1.00 | 3.97 |
| B4 | 0.98 | 0.00 | 0.98 | 1.00 | 3.99 |
| B5 | 0.95 | 0.03 | 0.98 | 1.00 | 3.98 |
| B6 | 0.96 | 0.02 | 0.98 | 1.00 | 4.00 |
| B7 | 0.98 | 0.00 | 0.98 | 1.00 | 3.97 |
| B8 | 0.92 | 0.05 | 0.97 | 1.00 | 3.98 |
| B9 | 0.97 | 0.00 | 0.97 | 1.00 | 3.98 |
| B10 | 0.93 | 0.04 | 0.97 | 1.00 | 3.97 |
| Average | 0.98 ± 0.01 | 3.98 ± 0.02 | |||
Image analysis of barytes for barium in barite and other minerals
Barytes is known to contain barium mainly as barite. However, qualitative EPMA results showed the presence of a barium aluminium silicate phase (barium feldspar) in four of the samples. Image analysis was carried out to assess the extent to which barium was present in minerals other than barite. Fig. 2 shows binerised X-ray images from barytes (B2). The white areas in the images correspond to: (1) total Ba; (2) total sulfur; (3) not-sulfur; (4) barium with not-sulfur. Table 4 gives the percent area of the image frame belonging to barium in the barite component and to barium in the feldspar. It can be seen that the bulk of the barium corresponds to barite. However, a small quantity of barium was found to be associated with silicon (and also with aluminium) which suggested the presence of barium feldspar in the commercial barytes. Barium feldspar with more than 90 percent of the BaAl2Si2O8 form is described as celsian whereas Ba-bearing feldspars with less than 30 percent of BaAl2Si2O8 are called hyalophane.11 The important consequence for the analytical chemist is that barytes containing barium feldspar may require a high temperature molten salt fusion to bring all the barium into solution. Higher concentrations of barium not associated with sulfur, in comparison with the barium associated with silicon (Table 4), may be due to the presence of BaCO3 in addition to barium feldspar in the barytes samples. | ||
| Fig. 2 X-ray images of barytes (B2); (1) Ba Lα , (2) S Kα , (3) not S, (4) Ba and (not S). | ||
| Barytes | Area (%) | Ba (%)a | Ba (%)b | ||
|---|---|---|---|---|---|
| Total Bac | Ba andd (not S) | Ba and Sie | Not in BaSO4 (from Ba, S) | Not in BaSO4 (from Ba, Si) | |
| a Calculated percentage of Ba which is not in the form of BaSO4 in barytes (values obtained by calculating the percentages of ‘b’ from Total Ba i.e. ‘a’.b Calculated percentage of Ba which is associated with Si (probably barium feldspar) in barytes (values obtained by calculating the percentage of ‘d’ from Total Ba i.e. ‘a’.c Total barium (% area) in image frame.d Barium (% area) which is not associated with S (i.e. Ba that is not in the form of BaSO4).e Area (%) of barium associated with Si. | |||||
| B1 | 73.6 | 14.6 | 5.6 | 19.9 | 7.6 |
| B2 | 84.2 | 7.7 | 3.7 | 9.1 | 4.4 |
| B3 | 95.2 | 14.7 | 5.6 | 15.4 | 5.8 |
| B4 | 51.5 | 1.0 | 0.1 | 2.0 | 0.3 |
| B5 | 86.5 | 0.4 | 0.4 | 0.4 | 0.8 |
| B6 | 70.8 | 1.6 | 1.9 | 2.2 | 2.6 |
| B7 | 78.8 | 1.7 | 2.4 | 2.1 | 3.1 |
| B8 | 73.5 | 5.2 | 1.0 | 7.0 | 1.4 |
| B9 | 78.7 | 1.3 | 3.0 | 1.7 | 3.8 |
| B10 | 81.6 | 5.7 | 2.3 | 7.0 | 2.9 |
Acid extractable trace elements in barytes
If the trace heavy metals in barytes are present in the associated minerals and if these are mainly sulfides (as seems likely from the EPMA results) then these metals should be acid extractable into an oxidising medium such as aqua regia, which, however, will dissolve only a small amount of barite (ca. 30 mg in 30 mL) and therefore not release trace metals in the barite lattice. ICP-MS was selected for a screening analysis for trace elements in aqua regia extracts of some barytes samples. A microwave-assisted aqua regia extraction was carried out to solubilise trace metals and the extract was diluted ten times before analysis. A large number of macro- and micro-elements were detected, including Ag, As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Sn, and Zn. Concentrations of those elements present at ‘high trace’ levels were determined by flame or by graphite furnace atomic absorption spectrometry (AAS), and are summarised in Table 5.| Metal | Barytes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 | B10 | |
| a Mean of triplicate measurements ± s; RSD (%) in parentheses. | ||||||||||
| Zn | 903 ± 25(3) | 1847 ± 6(0.3) | 4690 ± 17(1) | 67 ± 12(18) | 1303 ± 6(1) | 3513 ± 21(1) | 126 ± 20(16) | 59 ± 15(25) | 58 ± 4(7) | 3733 ± 42(1) |
| Pb | 414 ± 1(0.1) | 982 ± 20(2) | 3433 ± 137(4) | 27 ± 4(15) | 685 ± 11(2) | 68 ± 4(6) | 0.9 ± 0.4(46) | 931 ± 1(0.1) | 3.8 ± 0.4(11) | 3667 ± 98(3) |
| Cu | 22.0 ± 1.8(8) | 22.9 ± 1.0(4) | 82.4 ± 0.0(0) | 1.6 ± 0.0(0) | 22.6 ± 0.5(2) | 22.5 ± 2.7(12) | 4.2 ± 0.7(18) | 75.4 ± 1.6(2) | 1.2 ± 0.5(38) | 72.7 ± 3.2(4) |
| Fe | 12800 ± 256(2) | 20700 ± 207(1) | 28700 ± 287(1) | 4600 ± 322(7) | 48400 ± 484(1) | 30896 ± 974(3) | 4653 ± 159(3) | 28906 ± 939(3) | 7986 ± 159(2) | 45054 ± 980(2) |
| Mn | 170.5 ± 1.0(1) | 320.8 ± 3.9(1) | 104.4 ± 1.1(1) | 17.0 ± 1.1(7) | 143.5 ± 3.9(3) | 1668.4 ± 17(1) | 206.3 ± 4.0(2) | 3174.3 ± 2.0(1) | 191.7 ± 6.0(3) | 715.2 ± 20.0(3) |
| Hg | 3.90 ± 0.04(1) | 8.81 ± 0.56(6) | 24.85 ± 1.77(7) | 1.30 ± 0.10(8) | 1.60 ± 0.17(11) | 22.12 ± 1.54(7) | <0.04 | <0.04 | <0.04 | 3.72 ± 0.26(7) |
| As | 7.60 ± 0.39(5) | 35.36 ± 0.90(3) | 81.10 + 9.17(11) | 4.63 ± 0.44(10) | 9.03 ± 0.50(6) | 15.19 ± 0.52(4) | 1.16 ± 0.05(5) | 0.67 ± 0.07(10) | 5.08 ± 0.36(7) | 85.34 ± 3.90(5) |
| Cd | 0.85 ± 0.04(2) | 1.92 ± 0.26(8) | 23.60 ± 2.20(5) | 0.09 ± 0.02(13) | 0.76 ± 0.07(5) | 20.20 ± 2.00(6) | 0.23 ± 0.01(6) | 0.02 ± 0.02(100) | 0.06 ± 0.01(9) | 8.44 ± 0.53(3) |
Since metal sulfides can be dissolved by a strong oxidising acid attack, the analytical results obtained by this route should give the same values as a genuine ‘total analysis’, such as would be obtained from an aqua regia digestion with HF. Table 6 summarises the extent of agreement for these analyses using the two alternative digestions. They indicate that, except perhaps for copper where the HF combination extracts some 10% more, no additional amounts of these metals are being released when the silicate minerals are opened out. Some lead is probably being lost by precipitation when HF is used.
| Metal | |||||||
|---|---|---|---|---|---|---|---|
| As | Cu | Fe | Hg | Mn | Pb | Zn | |
| Slope, aqua regia/aqua regia–HF | 1.03 | 0.90 | 1.01 | 1.08 | 1.02 | 1.15 | 0.98 |
| Correlation coefficient, r | 0.94 | 0.98 | 0.99 | 0.83 | 0.99 | 0.99 | 0.99 |
The results in Table 5 serve to show, first, the ranges of concentrations of the metals found in a number of different samples (all marketed commercially) and, second, the precision achieved using this approach. A satisfactory precision is another indication that the method is well under control and therefore probably quantitative. Only for very low levels, such as copper in sample B9, were the RSDs high. The aqua regia digest together with the AAS measurement were validated by analysing a CRM, PACS-1, from the National Research Council of Canada, for which recoveries were 100 ± 5% for Cu, Fe, Hg, Pb, and Zn, with average RSD of 5%.
Patterns and similarities
Scatter diagrams for the concentrations of pairs of elements in the set of ten samples show clear separation into two groups: samples 1, 2, 3, 5, and 10, with higher concentrations of trace metals, and 4, 7 and 9, with much lower concentrations. Samples 6 and 8 did not fit either of these group patterns. The plot for copper against iron (Fig. 3) shows the clustering of data points clearly. The plot for copper against zinc, on the other hand, shows an excellent correlation when sample 8 is omitted (Fig. 4). This result prompted constructing the correlation matrix for all six of the major trace elements, shown in Table 7.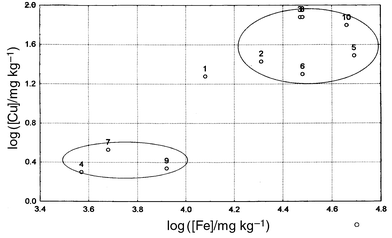 | ||
| Fig. 3 Scatter diagram for copper against iron in the barytes samples. Results are handled in logarithmic form for better visualisation. Note the two sub-groups. | ||
 | ||
| Fig. 4 Scatter diagram for copper against zinc in the barytes samples. Note the good linear correlation, with r2 = 0.98 over the whole set except for sample 8. | ||
| As | Cu | Fe | Mn | Pb | Zn | |
|---|---|---|---|---|---|---|
| a The values in bold indicate strong correlation of the pairs of elements. | ||||||
| As | 1 | |||||
| Cu | 0.71 | 1 | ||||
| Fe | 0.48 | 0.80 | 1 | |||
| Mn | 0.00 | 0.32 | 0.36 | 1 | ||
| Pb | 0.33 | 0.78 | 0.58 | 0.05 | 1 | |
| Zn | 0.69 | 0.93 | 0.45 | 0.03 | 0.37 | 1 |
Arsenic, copper and zinc correlate closely through the whole set of samples, with weaker links between copper and iron and copper and lead. It is interesting that the correlations become poorer when the low-level samples 4, 7 and 9 are omitted. The patterns of trace elements are therefore the same, even if the net concentrations are lower. It can now be pointed out, referring back to Table 2, that the sulfides of iron, lead and zinc were not seen by EPMA in this sub-group. On the other hand, this sub-group, 4, 7 and 9, tended to show greater predominance of silicate phases, both with and without barium.
Conclusions
Qualitative EPMA results have shown the presence of typically eight or more different minerals in the barytes samples, including barite, barium feldspar, galena, sphalerite, pyrite, quartz, silicates, etc. Quantitative EPMA confirmed that the barite (BaSO4) in the barytes often contains strontium (2–4% w/w) and a little calcium (<1%) as components of the crystal lattice. Image analysis shows that the bulk of the barium in barytes corresponds to the mineral barite (BaSO4). However, a small quantity of barium was found to be associated with silicon and aluminium, which confirmed the presence of barium feldspar. Of the major trace elements determined, arsenic, copper and zinc are closely correlated, all present in the sulfide phases. Lead is also correlated to the copper content, but less strongly to the other metals.The present study has shown clearly, using EPMA for direct analysis of the solids, and aqua regia digestion followed by AAS, as two independent analytical approaches, that the common heavy metals found in commercial barytes are present, not in the barite lattice, but in the associated minerals. These sulfide minerals can be brought into solution by digestion with aqua regia, without the need for dissolving the barite. The consequence is that routine analyses for monitoring the marine sediments around oil platforms can be carried out easily by an oxidising acid sample digestion with quantitation by ICP or AAS. It should be emphasised, however, that barium is not brought into solution except at very low concentrations, and this does not apply in the case of barytes samples. The results also suggest that selective solubilisation procedures for heavy metals in barytes would be useful for giving information regarding the bioavailability of heavy metals from barytes in marine pollution studies. Results from investigations into these two aspects are being prepared for publication.
Acknowledgements
The authors gratefully acknowledge the funding by EPSRC for the electron microprobe and wish to thank Dr J. M. S. Skakle (Department of Chemistry, University of Aberdeen) for her help in XRD analysis. T. M. Ansari is thankful to the Government of Pakistan and the Bahauddin Zakariya University, Multan, Pakistan, for the award of a Central Overseas Training Scholarship for PhD studies and the grant of a study leave abroad, respectively. We also gratefully acknowledge the assistance of Schlumberger Cambridge Research, Cambridge, and of the commercial drilling mud companies in Aberdeen, for providing some barytes samples.References
- C. Perricone, Proc. Symp. Res. Environ. Fate Eff. Drill. Fluids. Cutt., 1980, 2, 15. Search PubMed.
- Barite. Ch. in Mineral Commodity Summaries, USGS, Pittsburg, PA, 1999. Search PubMed.
- F. Olsgard and J. S. Gray, Mar. Ecol. Prog. Ser., 1995, 122, 277 CrossRef.
- S. H. B. Clark, M. J. Gallaghar and F. G. Poole, Trans. Instn. Min. Metall., 1990, 99, B125–132 CAS.
- D. A. Brobst, Trans. Instn. Min. Metall., 1984, 93, A123–130 Search PubMed.
- Mineral Resources Consultative Committee, Barium Minerals, HMSO, London, 1972, Mineral Dossier No.2. Search PubMed.
- L. G. Berry, B. Mason and R. V. Dietrich, Mineralogy, W. H. Freeman, San Francisco, CA, 2nd edn., 1983. Search PubMed.
- PARCOM, Convention for the Prevention of Marine Pollution from Land-based Sources (Paris Convention), OSPAR Commission, Amending Protocol, Paris, 1986. Search PubMed.
- US Environmental Protection Agency, Fed. Regist., 1997, 62, 1681 Search PubMed.
- McGraw-Hill Encyclopedia of Science & Technology, McGraw-Hill, New York, 7th edn., 1992. Search PubMed.
- W. A. Deer, R. A. Howie and J. Zussman, An introduction to the rock forming minerals, Longman, Hong Kong, 2nd edn., 1993 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |
