A comparison of sampling and analysis methods for low-ppbC levels of volatile organic compounds in ambient air†
E. Hunter
Daughtrey, Jr.
a,
Karen D.
Oliver
a,
Jeffrey R.
Adams
a,
Keith G.
Kronmiller
a,
William A.
Lonneman
b and
William A.
McClenny
c
aManTech Environmental Technology, Inc., P.O. Box 12313, Research Triangle Park, NC 27709, USA. E-mail: daughtrey.hunter@epamail.epa.gov; Fax: +1 919-541-3566
bSenior Environmental Employment Program, United States Environmental Protection Agency, 79 T.W. Alexander Drive, Research
Triangle Park, NC 27711, USA
cUnited States Environmental Protection
Agency, 79 T.W. Alexander Drive, Research Triangle Park, NC 27711, USA
First published on 11th January 2001
Abstract
A carefully designed study was conducted during the summer of 1998 to collect samples of ambient air by canisters and compare the analysis results to direct sorbent preconcentration results taken at the time of sample collection. Thirty-two 1 h sample sets were taken, each composed of a “near-real-time” sample analyzed by an autoGC-MS XonTech 930/Varian Saturn 2000 system, and Summa and Silco canisters. Hourly total non-methane organic carbon (TNMOC), ozone, and meteorological measurements were also made. Each canister was analyzed on the autoGC-MS system for a target list of 108 volatile organic compounds (VOCs) and on a manual cryosampling GC-FID system. Comparisons were made between the collection and analysis methods. Because of the low sample loading (150–250 ppbC TNMOC), these comparisons were a stringent test of sample collection and analysis capabilities. The following specific conclusions may be drawn from this study. Reasonable precision (within 15% mean difference of duplicate analyses from the same canister) can be obtained for analyses of target VOCs at low-ppbC concentrations. Relative accuracy between the GC-MS and GC-FID analysis methods is excellent, as demonstrated by comparisons of analyses of the same canisters, if measurements are sufficiently above the detection limits. This is especially significant as the GC-MS and GC-FID were independently calibrated. While statistically significant differences may be observed between the results from canister and near-real-time samples, the differences were generally small and there were clear correlations between the canister results and the near-real-time results. Canister cleanliness limits detection below the EPA Method TO-14 acceptance standard of 0.2 ppbv (0.2–2 ppbC for target analytes).
Introduction
Comparisons of sampling and analysis methods for volatile organic compounds (VOCs) of importance as potential ozone precursors were made at concentration levels typical of background sites. Cryogenic and sorbent sampling, and mass spectrometric (MS) and flame ionization detection (FID) of gas chromatographically separated VOCs were evaluated. The tests challenged both current method detection limits and canister cleanliness specifications. The results define attainable precision and accuracy near the detection limits.Many attempts have been made to evaluate data quality of trace-level VOC concentration measurements, but most fail to make the evaluation at ambient concentration levels. Most evaluations also require the use of canisters to provide a common source for multiple measurements, but their use has raised issues of canister cleanliness and artifacts.1,2 Round-robin analyses of canister-collected samples in large multiparticipant studies, such as the Non-methane Hydrocarbon Intercomparison Experiment (NOMHICE) and similar comparisons, often are difficult to interpret because of the variety of calibration and analysis methods, target lists, and the sheer size of the comparison.3 Sorbent sampling, as part of a preconcentration procedure for automated gas chromatographs, may also be subject to breakthrough and artifact issues, especially in the presence of humidity and ambient air oxidants, such as ozone.
Our laboratories have been in the forefront of making atmospheric VOC measurements by canister and near-real-time sorbent preconcentration, followed by gas chromatography (GC)-FID and GC-MS analysis.4–6 We define a “near-real-time” sample as one collected in 1 h and analyzed in the next hour with no use of intervening canister storage.
We designed our experiment to compare these sampling and analysis techniques by an especially rigorous scheme. The stringency of the design relates to the siting and seasonal timing of our measurements. At our sampling site in Research Triangle Park (RTP), NC, the expected ambient concentration levels for many analytes are near the detection limits for the methods or at the canister cleaning specifications. We had a target list of 108 analytes, most of which we expected to be present at concentrations less than the minimum detection limits for the sampling and analysis methods. We also investigated possible O3 interferences by arranging for the measurements to be made during the 1998 ozone season, both in the morning before breakup of the inversion layer and in the afternoon at the peak of ozone concentration. In this manner, we delineated a set of 32 × 1 h comparison periods for the study, comprising four sample sets per day for eight sampling days. Days predicted to have a high ozone concentration were selected for sampling. Actual ozone levels for the measurements ranged from 16 to112 ppb ozone and from 44 to 80% relative humidity.
Experimental method
Air was introduced into a heated (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C) glass sampling
manifold through a 3 m glass “candy cane”
(inlet 5 m
above ground level) with a measured flow of 368 L min−1
provided by a squirrel-cage blower. The airstream through the manifold
was sampled through 1/4 in ports to the ozone monitor (2 L min−1),
the autoGC-MS system (1 L min−1),
and through a dual canister sampler (0.32 L min−1)
into a Summa canister (either SIS, Moscow, ID; Graseby-Andersen,
Smyrna, GA; or BRC/Rasmussen, Hillsboro, OR) and a SilcoCan (Restek,
Bellefonte, PA).
°C) glass sampling
manifold through a 3 m glass “candy cane”
(inlet 5 m
above ground level) with a measured flow of 368 L min−1
provided by a squirrel-cage blower. The airstream through the manifold
was sampled through 1/4 in ports to the ozone monitor (2 L min−1),
the autoGC-MS system (1 L min−1),
and through a dual canister sampler (0.32 L min−1)
into a Summa canister (either SIS, Moscow, ID; Graseby-Andersen,
Smyrna, GA; or BRC/Rasmussen, Hillsboro, OR) and a SilcoCan (Restek,
Bellefonte, PA).
A commercially available XonTech Model 930 organic vapor concentrator (XonTech, Inc., Van Nuys, CA) equipped with two multisorbent traps was interfaced to a Saturn 2000 GC-MS (Varian Chromatography Systems, Walnut Creek, CA). All tubing and transfer lines were 1/16 in fused-silica-lined stainless-steel. The Model 930 two-trap system allowed the collection and dry purging of sample on one trap while the other trap was simultaneously cycled through the desorb, clean, and cool modes. The multisorbent traps contained 0.05 g Tenax-GR, 0.04 g Carbotrap, and 0.51 g Carbosieve S-III.7 The collected VOCs were desorbed from the Model 930 sorbent trap onto the GC column. We did not use the XonTech 940 Stirling engine focusing unit we have employed in previous studies,4 but only two target compounds (propane and chloromethane) were not focused sufficiently for chromatographic separation. A detailed description of the operation of the concentrator, cryotrap, and GC-MS system is given elsewhere.8 The system was calibrated by using dynamically diluted National Institute of Standards and Technology (NIST) traceable cylinder standards for the Photochemical Assessment Monitoring Station (PAMS) target list, selected terpenes, and the TO-14 target list (Air Liquide America, Houston, TX) and by an oxygenate standard prepared by dilution of aqueous cocktails of the neat materials. This autoGC-MS was used for both the near-real-time measurements and the GC-MS canister measurements, which were made 1–7 d after collection.
The cryosampling GC-FID system consists of a manual preconcentrator and a Hewlett-Packard Model 5890A Series II GC-FID system. The preconcentrator is a six-port gas-sampling valve configured with a 25 cm × 3.2 mm stainless-steel trap packed with 60–80 mesh untreated glass beads in place of a sampling loop. A more detailed system description is given elsewhere.9 Selected samples were analyzed with a similar trap and GC system by using an HP 5972 GC-MSD system. Calibration of the GC-FID was performed with a NIST propane Standard Reference Material. To compare the GC-FID results with the GC-MS results, effective carbon adjustment factors were used for the FID results.10,11
Results and discussion
In the 32 × 1 h measurement periods, we obtained data for 108 target analytes, most of which were below the detection limits. For purposes of discussion, a subset representative of various compound classes was chosen: (i) carbon tetrachloride, a ubiquitous atmospheric halocarbon at low concentration; (ii) isoprene and α-pinene, as biogenic hydrocarbons; (iii) benzene, toluene, and m,p-xylene, as automotive anthropogenic aromatics; and (iv) octanal as representative of n-aldehydes, suspected of having an artifact component to their analyzed concentrations.We compared the near-real-time sorbent preconcentration measurement results and the results of the analyses of samples collected in Summa and Silco canisters, both by sorbent preconcentration GC-MS and by cryofocused GC-FID. To put the measurements from this study in perspective, Table 1 presents a comparison of the maximum and average concentrations for selected VOCs from measurements made by this laboratory during field studies from 1995 to 1999, along with the method detection limit (MDL) for each compound. Detection limits are equal to or better than those presented by Brymer et al. in a canister storage stability study.12 It can readily be seen that the observed concentrations for this study were often much lower than those observed in the other studies and surveys by other workers13 and usually within an order of magnitude of the detection limit. This was due to the relatively low concentrations observed at RTP and the time of day and year of sampling.
| Compound | MDLa | This study | TN, 1995b | NC, 1995c | CA, 1997d | TN, 1999e | |
|---|---|---|---|---|---|---|---|
| a MDL = method detection limit; determined from analysis of seven replicates of 0.5 ppbv standard. b TN, 1995 = Southern Oxidants Study (SOS), New Hendersonville, TN, site, June 1995. c NC, 1995 = EPA ERC Annex parking lot, RTP, NC, summer 1995. d CA, 1997 = Southern California Ozone Study (SCOS97), Azusa, CA, September 1997. e TN, 1999 = Southern Oxidants Study (SOS), Cornelia Fort Airpark site, Nashville, TN, site, June–July 1999. f Max. = maximum observed concentration. g Avg. = mean observed concentration. h BDL = below detection limit. i Nearby clearing of pine trees yielded higher than normal concentrations. j ND = not determined. | |||||||
| Isoprene | 0.15 | Max.f | 14.9 | 10 | 16 | 8 | 28.5 |
| Avg.g | 8.0 | 2 | 3.5 | 1 | 2.3 | ||
| Benzene | 0.20 | Max. | 3.2 | 14 | 9.5 | 24 | 9.9 |
| Avg. | 0.9 | 2.5 | 2.5 | 8 | 3.2 | ||
| Carbon tetrachloride | 0.03 | Max. | 0.14 | 0.15 | 0.22 | 0.14 | 0.14 |
| Avg. | 0.12 | 0.08 | 0.10 | 0.11 | 0.11 | ||
| Toluene | 0.49 | Max. | 6.6 | 34 | 22 | 195 | 31 |
| Avg. | 2.4 | 4.7 | 3.0 | 28 | 4.9 | ||
| m,p-Xylene | 0.72 | Max. | 4.0 | 4.7 | 13 | 99 | 17 |
| Avg. | 1.0 | 1.0 | 1.9 | 14 | 2.5 | ||
| n-Decane | 0.80 | Max. | BDLh | 3.9 | 5.9 | 19 | 2.8 |
| Avg. | BDL | 0.6 | 0.8 | 2.7 | 0.5 | ||
| α-Pinene | 0.52 | Max. | 4.2 | 1.8 | 64i | 7.2 | 4.3 |
| Avg. | 1.7 | 0.6 | 7.1 | 1.1 | 0.3 | ||
| n-Octanal | NDj | Max. | 3.8 | 8.2 | 16.5 | 20 | 7.2 |
| Avg. | 2.4 | 3.6 | 5.8 | 4 | 3.6 | ||
Sampling by two types of canisters and in near-real-time plus analysis by both MS and FID offers more ways to plot the comparisons than could fit into a journal article. To conserve space, we have elected to present a single scatterplot and a table of the results of the other relevant comparisons for each of the compounds of interest. The raw data that led to these results are available (from the authors, on the Internet, and as microfiche).
The scatterplot for each compound (Fig. 1–7) plots the canister concentrations versus the near-real-time concentration for each of the two canister types. Legends were derived from the statistical analysis package used to generate the graphs and include the following abbreviations: SLMSAVG, the average mass spectrometric results from the analysis of a Silco canister sample; SMMSAVG, the average mass spectrometric results from the analysis of a Summa canister sample; x, the near-real-time GC-MS results; and eps, epsilon, the random error of the least-squares fit. The linear regression coefficients and the 95% confidence limits around the regression line (Silco: real-time, bold dashed line; Summa: real-time, bold solid line) are shown. Also noted on the graph are the MDL (vertical line) for the near-real-time measurements and the TO-14 canister cleanliness specification (horizontal line) for canister measurements. If an analysis artifact level has been determined,4 it is noted by a box.
A table for each compound (Tables 2–9) presents summary statistics and the results of t-tests for dependent samples. Comparisons are made for near-real-time MS measurements versus Silco canister MS measurements, Summa canister MS measurements, Silco FID results, and Summa FID results. Comparisons are also made for Silco MS results versus Summa MS and Silco FID results. Further comparisons are made between Summa MS and Summa FID results. For each sampling and analysis combination, the mean, the standard deviation (s), and the standard error of the mean (SEM), expressed both in terms of concentration (ppbC) and as a percentage of the mean, are determined. The standard deviation is a measure of the variation of observed concentrations over the 32 measurements. The SEM is a measure of measurement precision. For each of the sets of paired measurements, the difference and standard deviation of the difference (sdiff), again expressed both in terms of concentration and as a percentage of the mean, are given. The difference is a measure of how well the two sampling and analysis results agree, and the standard deviation is a measure of the overall precision. The t-statistic and the corresponding probability (p) are presented along with whether the difference is significant at the 95% confidence level.
Carbon tetrachloride
Carbon tetrachloride is reported to have a mean ambient air concentration of 0.1 ppb.13,14 We have observed no local sources at RTP, which is consistent with our observations in Alabama, Tennessee, and California. This level presents a stringent challenge for system detection limits, precision, and accuracy. The mean values found in the 32 sets of GC-MS analyses were 0.12 ppbv by near-real-time measurements, 0.12 ppbv in the Summa canisters, and 0.12 ppbv in the Silco canisters. No response is given for the GC-FID, as carbon tetrachloride does not readily burn in the FID flame. Measurements of precision are given in Table 2. The mean ratio of each of the canister results to the near-real-time was 0.97 for the Summa canisters and 0.99 for the Silco canisters; the precision of the ratios is consistent with the replicate precision measurements. No significant differences were seen between the measurements. Accuracy of all measurements relative to the reported global background level is within the measured precision. The net result is a confirmation that reasonable measurements can be made for both near-real-time and canister measurements at sub-ppb concentration levels, if no substrate (system) artifact or canister cleanup problems are present.| Variable | Mean (ppbC) | s (ppbC) | SEM (ppbC) | Difference (ppbC) | s diff (ppbC) | t | p | Sig? |
|---|---|---|---|---|---|---|---|---|
| Near-real-time | 0.120 | 0.012 (10%) | 0.0021 (1.7%) | 0.002 (1.7%) | 0.017 (14%) | 0.67 | 0.51 | N |
| Silco MS | 0.119 | 0.012 (10%) | 0.0022 (1.8%) | |||||
| Near-real-time | 0.121 | 0.012 | 0.0021 (1.7%) | 0.005 (4.2%) | 0.013 (11%) | 2.02 | 0.052 | N |
| Summa MS | 0.116 | 0.010 (9%) | 0.0018 (1.5%) | |||||
| Silco MS | 0.118 | 0.012 | 0.0021 (1.8%) | 0.002 (1.7%) | 0.013 (11%) | 0.94 | 0.352 | N |
| Summa MS | 0.116 | 0.010 | 0.0018 (1.5%) |
Biogenics
The choices of isoprene and α-pinene challenge the collection and analysis system in different ways. Isoprene has been a chief focus in the ozone precursor measurement community for many years.15 Isoprene showed morning and afternoon differences, as would be expected from its previously observed diurnal variation.4 The strong diurnal variation in α-pinene concentration was also expected from previous experience. Since α-pinene reaches its minimum during the daytime when the comparisons were conducted, this experiment gives a clear test of measurements near the detection limit.5A comparison of canister results to the near-real-time measurements for isoprene is given in Fig. 1 for both Summa and Silco canisters by GC-MS. All results are well above both the MDL and the canister cleanliness specification from TO-14. A clear linear relationship is evident between the results of canister samples (independent of type) and the near-real-time results. Isoprene results showed good precision, as shown by the standard error of the mean in Table 3, which ranged from 7.5% to 10%, while the measured concentrations varied by nearly an order of magnitude. Statistically significant differences were observed at the 95% confidence level between the near-real-time measurement and each of the canister methods (Silco, Summa, and FID). This indicates that there may be a slight loss of isoprene in canister collection. Storage stability studies performed in our laboratory at somewhat higher concentrations have shown reasonable stability, so any loss would have to occur in the few hours between sampling and analysis. The comparison between canister results (Silco vs. Summa and MS vs. FID for each canister) were not significantly different. This confirms the accuracy of the analyses. Another measure of relative accuracy is the mean ratio of FID ∶ MS results (expected value, 1.00), which was 1.00 ± 0.27 for Summa and 1.12 ± 0.44 for Silco.
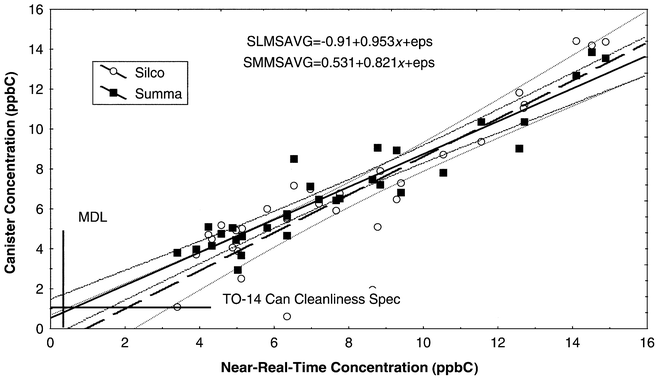 | ||
| Fig. 1 Scatterplot of isoprene canister concentration vs. near-real-time concentration, both in ppbC. | ||
| Variable | Mean (ppbC) | s (ppbC) | SEM (ppbC) | Difference (ppbC) | s diff (ppbC) | t | p | Sig? |
|---|---|---|---|---|---|---|---|---|
| Near-real-time | 7.97 | 3.38 (42%) | 0.60 (7.5%) | 1.29 (17%) | 1.68 (21%) | 4.33 | 0.00014 | Y |
| Silco MS | 6.68 | 3.63 (54%) | 0.64 (9.6%) | |||||
| Near-real-time | 7.82 | 3.38 (42%) | 0.60 (7.5%) | 0.87 (12%) | 1.14 (14%) | 4.23 | 0.00020 | Y |
| Summa MS | 6.96 | 2.90 (42%) | 0.52 (7.5%) | |||||
| Near-real-time | 7.85 | 3.38 (42%) | 0.60 (7.5%) | 1.03 (14%) | 2.58 (28%) | 2.22 | 0.03447 | Y |
| Silco FID | 6.83 | 3.68 (54%) | 0.66 (9.7%) | |||||
| Near-real-time | 7.97 | 3.38 (42%) | 0.60 (7.5%) | 0.88 (12%) | 2.23 (32%) | −4.38 | 0.00013 | Y |
| Summa FID | 7.09 | 3.30 (46%) | 0.58 (8.2%) | |||||
| Silco MS | 6.54 | 3.60 (54%) | 0.64 (9.6%) | −0.41 (−6%) | 1.76 (26%) | −1.29 | 0.205 | N |
| Summa MS | 6.96 | 2.90 (42%) | 0.52 (7.5%) | |||||
| Silco MS | 6.60 | 3.66 (54%) | 0.64 (9.6%) | −0.23 (−3%) | 2.43 (36%) | −0.53 | 0.600 | N |
| Silco FID | 6.83 | 3.68 (54%) | 0.66 (9.7%) | |||||
| Summa MS | 6.96 | 2.90 (42%) | 0.52 (7.5%) | −0.00 (0) | 1.92 (27%) | −0.006 | 0.995 | N |
| Summa FID | 6.96 | 3.27 (46%) | 0.58 (8.2%) |
For α-pinene, a clear difference was discernable between the morning and afternoon measurements, consistent with previously observed diurnal variation. The scatterplot of canister results versus near-real-time results, given in Fig. 2, shows both good linearity and agreement with a 1 : 1 line, despite most of the measurements being near the MDL and below the canister cleanliness specification. A plot for the canister FID results versus near-real-time measurements (not shown) yields similar slope and intercept, but slightly more scatter. This is also reflected in the FID ∶ MS ratio and its uncertainty, 0.84 ± 0.27 for Summa and 0.74 ± 0.44 for Silco. These ratios indicate a low-level nonspecific contaminant co-eluting with α-pinene, which raises the FID response at the low end of the concentration range proportionally more than at the high end where there is greater true signal. The results from each canister type show that 1 ppbC (0.1 ppbv) may be the smallest concentration discernable because of canister uncleanliness; this concentration is lower than the 0.2 ppbv TO-14 specification for canister cleanliness. This is reflected in the %RSD, given in Table 4, which is slightly larger than that observed for isoprene. Precision, as indicated by the standard error of the mean, was reasonable at 10–16%, despite the very low concentrations measured. The t-test for paired dependent samples shows no significant differences for most measurements. The significant difference observed between near-real-time measurements and Summa canisters may reflect the slight canister background. That it was significant for Summa and not for Silco may reflect the fact that the Summa canisters used in this study had been in use longer than the Silco canisters and may have had more residual VOCs contributing to canister background.
 | ||
| Fig. 2 Scatterplot of α-pinene canister concentration vs. near-real-time concentration, both in ppbC. | ||
| Variable | Mean (ppbC) | s (ppbC) | SEM (ppbC) | Difference (ppbC) | s diff (ppbC) | t | p | Sig? |
|---|---|---|---|---|---|---|---|---|
| Near-real-time | 1.74 | 1.07 (61%) | 0.23 (13%) | −0.24 (14%) | 0.49 (28%) | −2.10 | 0.05 | N |
| Silco MS | 1.98 | 1.18 (61%) | 0.19 (11%) | |||||
| Near-real-time | 1.74 | 1.07 (61%) | 0.23 (13%) | −0.32 (17%) | 0.33 (35%) | −4.28 | 0.0004 | Y |
| Summa MS | 2.06 | 1.15 (54%) | 0.19 (10%) | |||||
| Near-real-time | 1.80 | 1.07 (61%) | 0.23 (13%) | −0.02 (1%) | 0.60 (19%) | −0.11 | 0.914 | N |
| Silco FID | 1.82 | 1.20 (89%) | 0.21 (16%) | |||||
| Near-real-time | 1.71 | 1.05 (61%) | 0.23 (13%) | −0.16 (9%) | 0.73 (35%) | −0.97 | 0.341 | N |
| Summa FID | 1.87 | 1.04 (72%) | 0.18 (12%) | |||||
| Silco MS | 1.80 | 1.07 (61%) | 0.19 (11%) | −0.10 (5%) | 0.33 (17%) | −1.53 | 0.138 | N |
| Summa MS | 1.90 | 1.02 (54%) | 0.19 (10%) | |||||
| Silco MS | 1.73 | 1.06 (61%) | 0.19 (11%) | 0.41 (27%) | 0.81 (53%) | 2.68 | 0.012 | Y |
| Silco FID | 1.32 | 1.17 (89%) | 0.21 (16%) | |||||
| Summa MS | 1.85 | 1.00 (54%) | 0.19 (10%) | 0.33 (19%) | 0.79 (47%) | 2.20 | 0.036 | N |
| Summa FID | 1.52 | 1.07 (72%) | 0.18 (12%) |
Anthropogenic hydrocarbons
The aromatics and alkanes associated with vehicular activity were selected as indicator compounds in the evaluation as they are often the dominant VOCs in ambient air at our RTP test site. They show less diurnal variation than that observed for the above biogenic compounds; the aromatic concentrations do vary over about half an order of magnitude. Precision measurements of repeat analyses of canister results for the aromatics were consistent with other measurements at approximately 6–10% for the low-ppbC concentration range; the mean unsigned percentage difference between replicates for m,p-xylene was slightly elevated at 13% because of its lower concentration (0.5–4 ppbC).For benzene, canister results for both Summa and Silco are given in Fig. 3. What is most evident in this plot is the 2 ppbC benzene artifact previously noted4 with this sorbent preconcentration system. This yields high intercept values for the linear regression equations. Scatter of the data is reflective of most of the measurements being near the detection limit. Because the MS system employed a sorbent trap and the FID system used a cryogenic trap, we subtracted this 2 ± 0.2 ppbC artifact level from all of the MS results so that comparisons to the FID results would be valid. As given in Table 5, all differences among MS results were determined to be insignificant, while all measurements between MS results and FID results were shown to be significant. This probably indicates low-concentration interfering species in canisters co-eluting with the benzene peak in the FID analysis. Again, all concentrations are near the detection limit and canister cleanliness specification, indicated by the relatively low standard error of the mean, demonstrating reasonable analytical precision, and a large standard deviation of the differences, indicating larger sampling error.
 | ||
| Fig. 3 Scatterplot of benzene canister concentration vs. near-real-time concentration, both in ppbC. | ||
| Variable | Mean (ppbC) | s (ppbC) | SEM (ppbC) | Difference (ppbC) | s diff (ppbC) | t | p | Sig? |
|---|---|---|---|---|---|---|---|---|
| Near-real-time | 0.90 | 0.84 (93%) | 0.151 (17%) | −0.30 (−28%) | 1.08 (120%) | −1.527 | 0.137 | N |
| Silco MS | 1.20 | 0.99 (82%) | 0.263 (19%) | |||||
| Near-real-time | 0.90 | 0.84 (93%) | 0.151 (17%) | −0.11 (−11%) | 0.84 (92%) | −0.756 | 0.455 | N |
| Summa MS | 1.02 | 0.81 (79%) | 0.145 (14%) | |||||
| Near-real-time | 0.89 | 0.85 (93%) | 0.151 (17%) | −1.31 (−82%) | 1.40 (88%) | −5.13 | 0.000 | Y |
| Silco FID | 2.20 | 1.15 (54%) | 0.202 (9%) | |||||
| Near-real-time | 0.90 | 0.84 (93%) | 0.151 (17%) | −1.11 (−57%) | 1.13 (57%) | −5.44 | 0.000 | Y |
| Summa FID | 2.02 | 0.92 (45%) | 0.160 (8%) | |||||
| Silco MS | 1.20 | 0.99 (82%) | 0.263 (19%) | 0.18 (16%) | 0.51 (46%) | 1.99 | 0.055 | N |
| Summa MS | 1.02 | 0.81 (79%) | 0.145 (14%) | |||||
| Silco MS | 1.45 | 1.48 (101%) | 0.263 (19%) | −0.73 (−40%) | 1.58 (87%) | −2.60 | 0.0144 | Y |
| Silco FID | 2.19 | 1.13 (54%) | 0.202 (9%) | |||||
| Summa MS | 1.02 | 0.81 (79%) | 0.145 (14%) | −1.00 (−67%) | 0.97 (65%) | −5.70 | 0.000 | Y |
| Summa FID | 2.02 | 0.92 (45%) | 0.160 (8%) |
For toluene, the results of the comparison are shown in Fig. 4. A 0.5 ppbC positive bias was again observed for canisters relative to the near-real-time measurements, reflecting the difficulty of making measurements at or below the canister cleanliness specification. This concentration level is significantly below the 0.2 ppbv (1.4 ppbC) cleanliness specification level for TO-14, which is the basis for canister VOC measurements. No significant toluene artifact is seen for this sorbent combination, as indicated by both the FID versus near-real-time results and the results of the paired canisters. The mean FID ∶ MS ratio for the paired analyses was 1.26 ± 0.41 for Summa and 1.29 ± 0.61 for Silco. Table 6 shows that measurement precision is reasonable at 8–10% standard error of the mean for all measurements, indicating that the measured concentrations are well above the detection limit. Significant differences are observed for all measurements except the comparison of MS results between canister types, which suggests both a slight canister cleanliness issue and the previously noted nonspecific interferences seen with FID measurements.
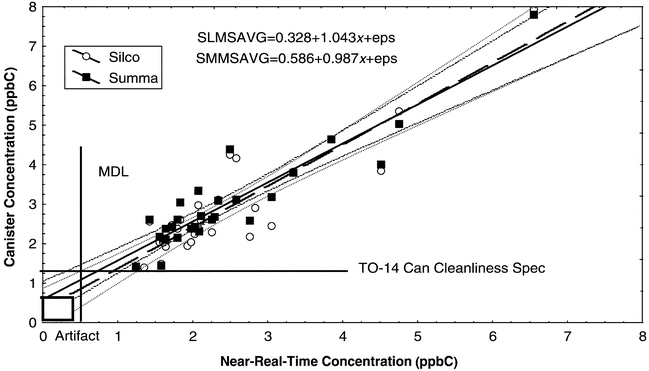 | ||
| Fig. 4 Scatterplot of toluene canister concentration vs. near-real-time concentration, both in ppbC. | ||
| Variable | Mean (ppbC) | s (ppbC) | SEM (ppbC) | Difference (ppbC) | s diff (ppbC) | t | p | Sig? |
|---|---|---|---|---|---|---|---|---|
| Near-real-time | 2.48 | 1.17 (47%) | 0.20 (8%) | −0.43 (−15%) | 0.59 (21%) | −4.00 | 0.000 | Y |
| Silco MS | 2.92 | 1.34 (46%) | 0.25 (8%) | |||||
| Near-real-time | 2.46 | 1.19 (47%) | 0.20 (8%) | −0.55 (−20%) | 0.49 (18%) | 6.00 | 0.000 | Y |
| Summa MS | 3.02 | 1.28 (42%) | 0.24 (8%) | |||||
| Near-real-time | 2.42 | 1.14 (47%) | 0.20 (8%) | −1.24 (−41%) | 1.30 (42%) | −5.00 | 0.000 | Y |
| Silco FID | 3.67 | 2.09 (57%) | 0.37 (10%) | |||||
| Near-real-time | 2.42 | 1.14 (47%) | 0.20 (8%) | −1.24 (−41%) | 1.12 (37%) | −6.00 | 0.000 | Y |
| Summa FID | 3.66 | 1.95 (53%) | 0.34 (9%) | |||||
| Silco MS | 3.02 | 1.38 (46%) | 0.25 (8%) | −0.08 (−3%) | 0.32 (10%) | −1.00 | 0.222 | N |
| Summa MS | 3.10 | 1.28 (42%) | 0.24 (8%) | |||||
| Silco MS | 2.92 | 1.35 (46%) | 0.25 (8%) | −0.76 (−23%) | 1.49 (45%) | 3.0 | 0.010 | Y |
| Silco FID | 3.68 | 2.10 (57%) | 0.37 (10%) | |||||
| Summa MS | 3.02 | 1.28 (42%) | 0.24 (8%) | −0.72 (−21%) | 1.40 (41%) | −3.0 | 0.013 | Y |
| Summa FID | 3.72 | 2.12 (53%) | 0.34 (9%) |
For m,p-xylene, the regression scatterplot, given in Fig. 5, shows that most of the measurements were at or near the detection limit. A higher intercept for the regression line for the Summa canister results probably indicates a higher background from more frequent usage, as has been previously noted. Table 7 shows slightly poorer measurement precision relative to toluene (7–16% standard error of the mean), reflective of the measured concentrations being at or near the detection limit. Significant differences were observed for all canister measurements relative to the near-real-time measurements. Comparisons between canister measurements showed no significant differences; this good agreement validates the measurement accuracy.
 | ||
| Fig. 5 Scatterplot of m,p-xylene canister concentration vs. near-real-time concentration, both in ppbC. | ||
| Variable | Mean (ppbC) | s (ppbC) | SEM (ppbC) | Difference (ppbC) | s diff (ppbC) | t | p | Sig? |
|---|---|---|---|---|---|---|---|---|
| Near-real-time | 1.32 | 0.80 (60%) | 0.14 (11%) | −0.90 (−51%) | 0.57 (32%) | −9 | 0.000 | Y |
| Silco MS | 2.22 | 0.85 (38%) | 0.15 (7%) | |||||
| Near-real-time | 1.32 | 0.80 (60%) | 0.14 (11%) | −0.95 (−53%) | 0.79 (44%) | −7 | 0.000 | Y |
| Summa MS | 2.27 | 0.85 (37%) | 0.15 (7%) | |||||
| Near-real-time | 1.35 | 0.84 (60%) | 0.14 (11%) | −0.98 (−53%) | 1.69 (92%) | −6 | 0.000 | Y |
| Silco FID | 2.32 | 1.96 (16%) | 0.37 (16%) | |||||
| Near-real-time | 1.32 | 0.80 (60%) | 0.14 (11%) | −0.66 (−39%) | 0.59 (35%) | −6 | 0.000 | Y |
| Summa FID | 1.98 | 1.13 (57%) | 0.20 (10%) | |||||
| Silco MS | 2.22 | 0.85 (38%) | 0.15 (7%) | −0.05 (−2%) | 0.82 (36%) | −0.0 | 0.698 | N |
| Summa MS | 2.27 | 0.85 (37%) | 0.15 (7%) | |||||
| Silco MS | 2.26 | 0.88 (38%) | 0.15 (7%) | −0.06 (−3%) | 1.81 (79%) | −0.00 | 0.85 | N |
| Silco FID | 2.32 | 1.95 (16%) | 0.37 (16%) | |||||
| Summa MS | 2.27 | 0.85 (37%) | 0.15 (7%) | 0.29 (13%) | 1.04 (48%) | 2 | 0.119 | N |
| Summa FID | 1.98 | 1.13 (57%) | 0.20 (10%) |
Analysis of n-decane was included to test performance at very low concentration levels. The scatterplot in Fig. 6 shows that the canister results have little correlation to the near-real-time concentration results and that all measurements were below both the detection limit and the cleanliness standard. No results were obtained for FID measurements, as the signal was below the peak threshold trigger. Table 8 yields standard errors of the mean ranging from 4% to 16%, with significant differences between canister and near-real-time results. Because the results are below the detection limit, no conclusions should be drawn as to the validity of the differences.
 | ||
| Fig. 6 Scatterplot of n-decane canister concentration vs. near-real-time concentration, both in ppbC. | ||
| Variable | Meana (ppbC) | s (ppbC) | SEM (ppbC) | Difference (ppbC) | s diff (ppbC) | t | p | Sig? |
|---|---|---|---|---|---|---|---|---|
| a ND = not determined. | ||||||||
| Near-real-time | 0.23 | 0.07 (30%) | 0.01 (4%) | −0.58 (−111%) | 0.68 (131%) | −5 | 0.000 | Y |
| Silco MS | 0.81 | 0.71 (87%) | 0.12 (15%) | |||||
| Near-real-time | 0.23 | 0.07 (30%) | 0.01 (4%) | −0.52 (−106%) | 0.25 (51%) | −11 | 0.000 | Y |
| Summa MS | 0.75 | 0.28 (37%) | 0.12 (16%) | |||||
| Near-real-time | ||||||||
| Silco FID | ND | |||||||
| Near-real-time | ||||||||
| Summa FID | ND | |||||||
| Silco MS | 0.79 | 0.69 (87%) | 0.12 (15%) | 0.04 (5%) | 0.64 (83%) | −1 | 0.75 | N |
| Summa MS | 0.75 | 0.28 (37%) | 0.12 (16%) | |||||
| Silco MS | ||||||||
| Silco FID | ND | |||||||
| Summa MS | ||||||||
| Summa FID | ND | |||||||
n-Aldehydes
The n-aldehydes have been the subject of a great deal of debate as to whether they are really in the atmosphere or are an artifact of sampling or analysis.5,16–19 The results found here, as shown in Fig. 7, do show a large difference and little correlation between the near-real-time results and the canister analyses, with the canister results generally being higher than the near-real-time results. This is further confirmed in the analysis of paired samples. The precision, as given by the standard error of the mean (8–22%) shown in Table 9, demonstrates greater variability than that exhibited for the other selected compounds. All measured differences were statistically significant except the Silco versus Summa MS results, even at this higher scatter. This can be explained as residual aldehydes in the canisters, for which there is evidence in the experience of both this laboratory and others.6,19 An alternative explanation is that ozone in the air may destroy octanal in the near-real-time sample collected with the sorbent trap, whereas ozone wall losses in the canister prevent octanal losses in the canister samples. The persistence of aldehydes, even in humidified cleaned canisters, favors the former explanation. For all of the studied compounds except octanal, we believe that adequate explanations are given for the observed differences. For octanal, the differences are larger, but much more difficult to explain. Octanal in the in situ measurement does scale somewhat with the ozone concentration, but the canister octanal measurements do not. Because of the difficulty in discerning effects at or near the detection limits in the presence of a significant artifact, additional investigations are being performed in our laboratory with augmented concentrations of oxygenated compounds and ozone. | ||
| Fig. 7 Scatterplot of n-octanal canister concentration vs. near-real-time concentration, both in ppbC. | ||
| Variable | Mean (ppbC) | s (ppbC) | SEM | Difference | s diff | t | p | Sig? |
|---|---|---|---|---|---|---|---|---|
| Near-real-time | 0.68 | 0.83 (122%) | 0.15 (22%) | −1.28 (93%) | 2.08 (152%) | −8 | 0.000 | Y |
| Silco MS | 1.96 | 2.26 (115%) | 0.41 (21%) | |||||
| Near-real-time | 0.69 | 0.82 (119%) | 0.15 (22%) | −0.81 (−74%) | 1.27 (115%) | −4 | 0.001 | Y |
| Summa MS | 1.50 | 1.44 (96%) | 0.25 (17%) | |||||
| Near-real-time | 0.72 | 0.82 (114%) | 0.15 (21%) | −2.47 (127%) | 2.23 (132%) | −6 | 0.000 | Y |
| Silco FID | 3.19 | 2.39 (75%) | 0.42 (13%) | |||||
| Near-real-time | 0.70 | 0.82 (117%) | 0.15 (21%) | −2.16 (121%) | 1.43 (80%) | −8 | 0.000 | Y |
| Summa FID | 2.86 | 1.40 (50%) | 0.24 (8%) | |||||
| Silco MS | 1.94 | 2.22 (114%) | 0.41 (21%) | 0.45 (26%) | 2.32 (135%) | 1 | 0.297 | N |
| Summa MS | 1.49 | 1.44 (97%) | 0.25 (17%) | |||||
| Silco MS | 1.94 | 2.22 (114%) | 0.41 (21%) | −0.95 (−39%) | 1.17 (82%) | 3 | 0.002 | Y |
| Silco FID | 2.89 | 1.74 (60%) | 0.42 (14%) | |||||
| Summa MS | 1.51 | 1.42 (94%) | 0.25 (16%) | −1.33 (61%) | 1.57 72%) | −5 | 0.000 | Y |
| Summa FID | 2.85 | 1.36 (48%) | 0.24 (8%) |
Conclusions
The following specific conclusions may be drawn from this study.Reasonable precision (within 15% mean difference of duplicate analyses from the same canister) can be obtained for analyses of target VOCs at low-ppbC concentrations.
Relative accuracy between the GC-MS and GC-FID analysis methods is excellent, as demonstrated by comparisons of analyses of the same canisters, if measurements are sufficiently above the detection limits. This is especially significant as the GC-MS and GC-FID were independently calibrated—the GC-MS by multicomponent VOC standards and the GC-FID by NIST-traceable propane with correction factors applied for oxygenated species.
While statistically significant differences may be observed between the results from canister and near-real-time samples, the differences were generally small and there were clear correlations between the canister results and the near-real-time results.
Canister cleanliness limits detection below the TO-14 acceptance standard of 0.2 ppbv (0.2–2 ppbC for target analytes). Further improvements in canister cleaning techniques are being investigated by our laboratory by use of oil-free vacuum pumps and different heating and steam cleaning approaches.
Canister results may be biased slightly high for PAMS target compounds because of the limit of canister uncleanliness.
Aldehyde collection and analysis may be precluded in canisters unless a much improved cleaning procedure can be developed and demonstrated.
Acknowledgements
The U.S. Environmental Protection Agency through its Office of Research and Development partially funded and managed the research described here under Contract 68-D5-0049 to ManTech Environmental Technology, Inc. It has been subject to Agency review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.References
- W. A. McClenny and N. O. Gerald, Environ. Lab., 1994, 60, 37 Search PubMed.
- N. O. Gerald and J. Rice, in Proceedings of the 1998 EPA/A&WMA International Conference on Air Toxics and Related Air Pollutants, Research Triangle Park, NC, Air & Waste Management Association, Pittsburgh, PA, pp. 959–966. Search PubMed.
- E. C. Apel, J. G. Calvert and F. C. Fehsenfeld, J. Geophys. Res., 1994, 99, 16651 CrossRef CAS.
- E. H. Daughtrey, Jr., J. R. Adams, K. D. Oliver, K. G. Kronmiller and W. A. McClenny, J. Geophys. Res. Atmos., 1998, 103, 22375 Search PubMed.
- W. A. McClenny, E. H. Daughtrey, Jr., J. R. Adams, K. D. Oliver and K. G. Kronmiller, J. Geophys. Res. Atmos., 1998, 103, 22509 Search PubMed.
- E. C. Apel, J. G. Calvert, D. Reimer, W. Pos, R. Zika, T. E. Kleindienst, W. A. Lonneman, K. Fung, E. Fujita, P. B. Shepson, T. K. Starn and P. T. Roberts, J. Geophys. Res. Atmos., 1998, 103, 22295 Search PubMed.
- D. A. Levaggi, W. Oyung and R. Zerrudo, in Proceedings of the 1992 U.S. EPA/A&WMA International Symposium on Measurement of Toxic and Related Air Pollutants, VIP-25, Air & Waste Management Association, Pittsburgh, PA, 1992, pp. 857–863. Search PubMed.
- K. D. Oliver, J. R. Adams, E. H. Daughtrey, Jr., W. A. McClenny, M. J. Yoong, M. A. Pardee, E. B. Almasi and N. A. Kirshen, Environ. Sci. Technol., 1996, 30, 1939 CrossRef CAS.
- W. A. Lonneman, in Proceedings of the 1998 EPA/A&WMA Symposium on Measurement of Toxic and Related Air Pollutants, Air & Waste Management Association, Pittsburgh, PA, pp. 356–365. Search PubMed.
- A. D. Jorgensen, K. C. Picel and V. C. Stamoudis, Anal. Chem., 1990, 62, 683 CrossRef CAS.
- J. T. Scanlon and D. E. Willis, J. Chromatogr. Sci., 1985, 23, 333 CAS.
- D. A. Brymer, L. D. Ogle, C. J. Jones and D. L. Lewis, Environ. Sci. Technol., 1996, 30, 188 CrossRef CAS.
- J. J. Shah and H. B. Singh, Environ. Sci. Technol., 1988, 22, 1381 CAS.
- P. Warneck, Chemistry of the Natural Atmosphere, Academic Press, San Diego, 1988, p. 270. Search PubMed.
- A. P. Altshuller, Atmos. Environ., 1983, 17, 2131 CrossRef CAS.
- E. H. Daughtrey, Jr., J. R. Adams, K. G. Kronmiller and K. D. Oliver, in Proceedings of the 1998 EPA/A&WMA Symposium on Measurement of Toxic and Related Air Pollutants, Air & Waste Management Association, Pittsburgh, PA, pp. 851–861. Search PubMed.
- T. K. Starn, P. B. Shepson, S. B. Bertman, J. S. White, B. G. Splawn, D. D. Reimer, R. G. Zika and K. Olszyna, J. Geophys. Res., 1998, 103, 22425 CrossRef CAS.
- T. K. Starn, P. B. Shepson, S. B. Bertman, D. D. Reimer, R. G. Zika and K. Olszyna, J. Geophys. Res., 1998, 103, 22437 CrossRef CAS.
- S. A. Batterman, G.-Z. Zhang and M. Baumann, Atmos. Environ., 1998, 32, 1647 CrossRef CAS.
Footnote |
| † This is the work of United States government employees engaged in their official duties. As such it is in the public domain and exempt from copyright. ©US government. |
| This journal is © The Royal Society of Chemistry 2001 |
