The first example of an anion–pyrrole complex
Simon J.
Coles
,
Philip A.
Gale
* and
Michael B.
Hursthouse
Department of Chemistry, University of Southampton, Southampton, UK SO17 1BJ. E-mail: philip.gale@soton.ac.uk
Abstract
Tetramethylammonium chloride (1) has been recrystallized from pyrrole (2) yielding a pyrrole–chloride complex (I) that has been crystallographically characterized. Complex I contains a chloride anion that is bound by hydrogen bonds from two pyrrole rings and a tetramethylammonium cation. The supramolecular structure is reminiscent of a honeycomb array, comprised of a pyrrole–chloride framework with cavities occupied by tetramethylammonium moities.
Introduction
The supramolecular chemistry of anions in solution1 and in the solid state,2 and in particular their molecular recognition properties and role in self-assembly processes,3 is an area of continuing interest and research effort. Pyrrolic and poly-pyrrolic anion receptors are an important subset of anion recognition agents. These systems may be charged4 or neutral5 and coordinate anions via hydrogen bond donation from a number of pyrrole rings6 or from pyrrole in combination with other hydrogen bond donor groups.7 Whilst these receptors are extremely efficient and selective in their anion binding properties, simple pyrroles interact only weakly with anions in solution.8 In order to prepare an anion complex of pyrrole itself we have crystallized tetramethylammonium chloride from pyrrole. The resulting complex has been crystallographically characterized, revealing the formation of pyrrole NH⋯anion hydrogen bonds in the solid state. Whilst the crystal structure of pyrrole reveals the formation of NH⋯π hydrogen bonds,9 we believe this is the first time a hydrogen bonded anion complex of a single pyrrole ring has been synthesized and characterized.The asymmetric unit of structure I, shown in Fig. 1, is comprised of a single pyrrole, co-crystallized with half a chloride anion and a partial tetramethylammonium cation situated on the mirror plane of the space group.
 | ||
| Fig. 1 Asymmetric unit and numbering scheme of I (i = −x, 1 − y, z − 1/2). | ||
The supramolecular structure of the crystal depicted in Fig. 2 shows the chloride anion to be hydrogen bonded to two pyrrole heterocycles, in addition to a presumably weaker interaction to the tetramethylammonium cation, as demonstrated by the intermolecular separations in Table 1.
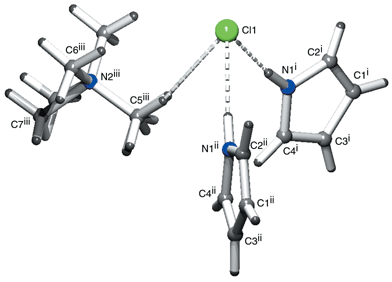 | ||
| Fig. 2 Coordination environment around the chloride anion (i = 1/2 − x, 1/2 − y, 1/2 + z; ii = −1/2 + x, 1/2 − y, 1/2 + z; iii = x, 1 − y, 1/2 + z). | ||
| D–H⋯A | d(D–H)/Å | d(H⋯A)/Å | d(D⋯A)/Å | ∠(D–H⋯A)/° |
|---|---|---|---|---|
| a Symmetry operations: i = 1 − x, 2 − y, −1/2 + z; ii = x + 1/2, −y + 3/2, z + 1/2; iii = 1/2 − x, 1/2 − y, 1/2 + z. | ||||
| N1–H1N⋯Cl1i | 0.918(4) | 2.327(4) | 3.241(3) | 174(4) |
| C5ii–H5Aii⋯Cl1 | 0.980 | 2.713(4) | 3.656(7) | 161(3) |
| C7–H7B⋯Centroidiiipyrrole | 0.980 | 2.600(5) | 3.433(5) | 143(4) |
Comparison of the pyrrole–chloride interactions in I with those observed in the chloride complex of meso-octamethylcalix[4]pyrrole8 (CCDC code = TEQKIJ9) reveals a marked similarity in the donor–acceptor separation [i.e. it is in the range 3.264(7)–3.331(7) Å in the macrocyclic complex and is 3.241(9) Å in I] and also in the angle between adjacent donors and the anion [in meso-octamethylcalix[4]pyrrole it is 60.36(8)°, compared to 61.73(15)° in I]. Furthermore, a C–H⋯π interaction is observed between a methyl proton of the tetramethylammonium group and the ring centroid of the pyrrole, a similar interaction to the NH⋯π hydrogen bond observed in the crystal structure of pyrrole10 and a CH⋯π interaction in the dichloromethane solvate of meso-tetraspirocyclohexylcalix[4]pyrrole.8
The dihedral angle of 61.73(15)° allows the two coordinating pyrrole units to form a cleft in which the tetramethylammonium group is accommodated. Investigation of the supramolecular structure of the extended solid shows the tetramethylammonium molecules to be positioned between two pyrrole–chloride–pyrrole complex clefts, giving rise to a structure reminiscent of a honeycomb framework depicted in Fig. 3. When viewed parallel to the c axis, the cavities produced by the encapsulating pyrrole framework are occupied by two tetramethylammonium moieties arranged in a head-to-tail fashion.
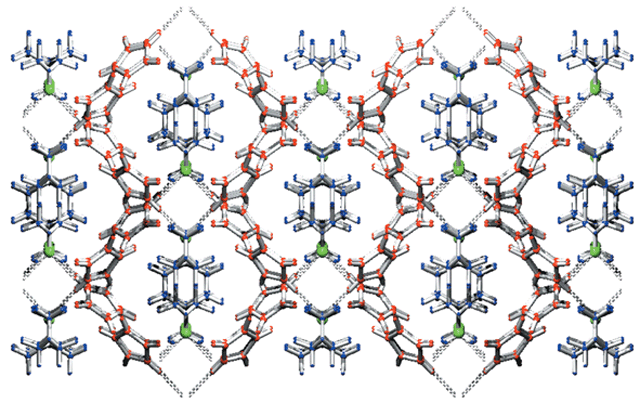 | ||
| Fig. 3 Honeycomb-like array, parallel to the c axis, adopted by the supramolecular structure. Colour code: pyrrole red, tetramethylammonium blue, chloride green. Click image or 3.htm to access a 3D representation. (The 3D structure shows a degree of disorder for the tetramethylammonium moieties.) | ||
Experimental
Tetramethylammonium chloride 1 and pyrrole 2 were purchased from the Aldrich Chemical company. Pyrrole was freshly distilled and then a saturated solution of 1 in pyrrole was prepared and stored in a desiccator. Crystals formed over a matter of weeks and were analyzed by single crystal X-ray diffraction.Crystallographic data collection and structure determination
Single crystal diffraction data were collected on a Nonius KappaCCD diffractometer equipped with a Nonius FR591 rotating anode X-ray generator. The structures were solved and refined using the SHELX11 suite of programs. Protons were included in idealized positions with their thermal parameters riding on the values of the parent atom. The tetramethylammonium moiety lies on a mirror plane such that atoms C5 and C7 exhibit crystallographic disorder, and the occupancies of the unique atoms have accordingly been set at 50%. Brief details of the data collection and structure refinement are summarized in Table 2.| Parameter | |
|---|---|
| a Click b109813f.txt for full crystallographic data (CCDC 173101). | |
| Asymmetric unit formula | C6H11Cl0.5N1.5 |
| Crystal dimensions/mm | 0.24 × 0.2 × 0.04 |
| M | 121.89 |
| Crystal morphology | Plate (colourless) |
| Crystal system | Orthorhombic |
| Space group | Cmc21 |
| a/Å | 15.094(2) |
| b/Å | 9.007(2) |
| c/Å | 10.325(6) |
| V/Å3 | 1403.7(4) |
| Z | 8 |
| T/K | 120 |
| D c/Mg m−3 | 1.154 |
| λ/Å | 0.71073 |
| Absorption coefficient, μ/mm−1 | 0.253 |
| θ Range/° | 2.63–27.49 |
| Measured reflections | 3927 |
| Independent reflections | 1465 |
| R int | 0.0851 |
| R indices [F2 > 2σ(F2)] | R 1 = 0.0617 |
| wR 2 = 0.1393 | |
| R indices (all data) | R 1 = 0.0900 |
| wR 2 = 0.1558 | |
| Absolute structure parameter | −0.14(13) |
| Δρmax., Δρmin./e Å−3 | 0.521, −0.416 |
Conclusion
Complex I is, to the best of our knowledge, the first example of a single pyrrole ring–anion complex to be reported.12 The similarities in the geometry of the pyrrole–chloride interactions in I with the N–H⋯Cl− geometry in the chloride complex of meso-octamethylcalix[4]pyrrole sheds some light upon the remarkable anion complexation properties of these macrocycles in that without macrocyclic constraints, simple pyrrole adopts a very similar coordination geometry.Acknowledgements
The authors would like to thank the EPSRC for funding of crystallographic facilities, whilst P. A. G. thanks the Royal Society for a University Research Fellowship.References
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef; M. M. G. Antonisse and D. N. Reinhoudt, Chem. Commun., 1998, 443 RSC; The Supramolecular Chemistry of Anions, ed. A. Bianchi, A. K. Bowman-James and E. Garcia-España, Wiley-VCH, New York, 1997 Search PubMed; F. P. Schmidtchen and M. Berger, Chem. Rev., 1997, 97, 1609 Search PubMed; J. L. Atwood, K. T. Holman and J. W. Steed, Chem. Commun., 1996, 1401 CrossRef CAS; A. P. Davis, J. F. Gilmer and J. J. Perry, Angew. Chem., Int. Ed. Engl., 1996, 35, 1312 RSC; J. L. Sessler and W. E. Allen, CHEMTECH, 1999, 29, 16 CrossRef CAS.
- M. E. Light, S. Camiolo, P. A. Gale and M. B. Hursthouse, Acta Crystallogr., Sect. E, 2001, 57, o727 CrossRef; C. A. Iloudis, K. S. B. Hancock, D. G. Georganopoulou and J. W. Steed, New J. Chem., 2000, 787 RSC.
- P. A. Gale, Coord. Chem. Rev., 2000, 199, 181 CrossRef CAS; P. A. Gale, Coord. Chem. Rev., 2001, 213, 79 CrossRef CAS.
- J. L. Sessler and W. E. Allen, CHEMTECH, 1999, 29, 16 CAS; J. L. Sessler and S. J. Weghorn, Expanded, Contracted and Isomeric Porphyrins, Elsevier, Oxford, 1997 Search PubMed.
- J. L. Sessler and P. A. Gale, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, San Diego, CA and Burlington, MA, 2000, vol. 6, pp. 257–278 Search PubMed.
- P. A. Gale, J. L. Sessler and V. Král, Chem. Commun., 1998, 1 RSC.
- P. A. Gale, S. Camiolo, G. J. Tizzard, C. P. Chapman, M. E. Light, S. J. Coles and M. B. Hursthouse, J. Org. Chem., 2001, 66, 7849 CrossRef CAS; P. A. Gale, S. Camiolo, C. P. Chapman, M. E. Light and M. B. Hursthouse, Tetrahedron Lett., 2001, 42, 5095 CrossRef CAS; A. Andrievsky, F. Ahuis, J. L. Sessler, F. Vögtle, D. Gudat and M. Moini, J. Am. Chem. Soc., 1998, 120, 9712 CrossRef CAS; C. Schmuck, Chem. Eur. J., 2000, 6, 709 CrossRef CAS; C. Schmuck, Chem. Commun., 1999, 843 RSC.
- P. A. Gale, J. L. Sessler, V. Král and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140 CrossRef CAS.
- F. H. Allen and O. Kennard, Chem. Des. Automat. News, 1993, 8, 31 Search PubMed.
- R. Goddard, O. Heinemann and C. Krügger, Acta Crystallogr., Sect. C, 1997, 53, 1846 CrossRef.
- G. M. Sheldrick, SHELXS97 and SHELXL97, Programs for Crystal Structure Solution and Refinement, University of Göttingen, Germany, 1997.
- G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond, Oxford University Press and the International Union of Crystallography, Oxford, 1999 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |
