[Et4N]2[TCNE]2 (TCNE = tetracyanoethylene) – an example of an exceptionally long 2.827 Å CC bond
Rico E.
Del Sesto
a,
Roger D.
Sommer
b and
Joel S.
Miller
*a
aDepartment of Chemistry, University of Utah, 315 So. 1400 E. Rm. 2124, Salt Lake City, UT 84112-0850, USA. E-mail: jsmiller@chemistry.utah.edu
bDepartment of Chemistry, Colorado State University, Fort Collins, CO 80523, USA
Abstract
The reaction of [Et4N]I and tetracyanoethylene (TCNE) forms [Et4N]2[TCNE]2, which possesses [TCNE]22− with a 2.827(3) Å intradimer CC bond distance and exhibits νC![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) N at 2191, 2170 and 2163 cm−1, νC
N at 2191, 2170 and 2163 cm−1, νC![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) C at 1365 cm−1, and UV-Vis bands at 26
C at 1365 cm−1, and UV-Vis bands at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 and 16
150 and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 cm−1.
850 cm−1.
Introduction
Interest in organic compounds exhibiting unusually long CC bonds has been the subject of several recent studies.1–3 The longest sp3–sp3 C–C single bond reported to date is 1.73 Å,1,2 whereas several [TCNE]22− (TCNE = tetracyanoethylene) dimers have been recently reported to form cation-assisted, long, two-electron π*–π* CC bonds involving four carbon atoms. These CC bonds range from 2.833 to 3.09 Å. The cations range from electrostatically bonded Tl+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and K+
4 and K+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to large, bulky, non-coordinating cations such as [Cr(C6H6)2]+,6
[Fe(C5H4)2C3H6]+,7 and [TDAE]2+
[TDAE = (Me2N)2CC(NMe2)2].4,8 Herein we report the structure, IR and UV-Vis spectroscopic properties of [Et4N]2[TCNE]2, a new example of the [TCNE]22− dimer, which exhibits the shortest π*–π* CC bond for this family of compounds.
5 to large, bulky, non-coordinating cations such as [Cr(C6H6)2]+,6
[Fe(C5H4)2C3H6]+,7 and [TDAE]2+
[TDAE = (Me2N)2CC(NMe2)2].4,8 Herein we report the structure, IR and UV-Vis spectroscopic properties of [Et4N]2[TCNE]2, a new example of the [TCNE]22− dimer, which exhibits the shortest π*–π* CC bond for this family of compounds.
Experimental
Synthesis
A solution of [Et4N]I [385 mg (1.5 mmol) in 10 mL hot MeCN] was slowly added to a rapidly stirring solution of TCNE [128 mg (1.0 mmol) in 5 mL of hot MeCN]. The solution was allowed to stir for one hour, and then placed in a freezer at −30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. The resulting crystals were filtered out and recrystallized twice more from MeCN. IR (KBr/cm−1): νC
°C. The resulting crystals were filtered out and recrystallized twice more from MeCN. IR (KBr/cm−1): νC![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) N 2191 (m), 2170 (s), 2163 (s); νC
N 2191 (m), 2170 (s), 2163 (s); νC![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) C 1365 (s). UV-Vis (KBr/cm−1): 26
C 1365 (s). UV-Vis (KBr/cm−1): 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150, 16
150, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850.
850.
Crystal structure determination
A crystalline sample of [Et4N][TCNE] was prepared by slow evaporation of a MeCN solution under nitrogen. A suitable crystal was selected and mounted on a thin glass fiber using silicone grease. Preliminary unit-cell determinations were obtained by harvesting reflections from three orthogonal sets of 20 frames, using −0.3° ω scans. These results were confirmed by refinement of unit-cell parameters during integration. Crystallographic information is summarized in Table 1. The structure was solved using direct methods. Non-hydrogen atoms were located by difference Fourier synthesis and were refined anisotropically. Hydrogen atoms were added at calculated positions and treated as isotropic contributions with thermal parameters defined as 1.2 or 1.5 times that of the parent atom. The ethyl groups of the [Et4N]+ are unequally disordered over two orientations (vide infra). All software and sources of scattering factors are contained in the SHELXTL-97 program library (version 5.10, G. Sheldrick, Bruker-AXS, Madison, WI, 1997).| Parameter | |
|---|---|
a Click b108455k.txt for full crystallographic data (CCDC 170964).
b
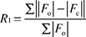 ; ; 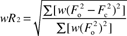 .
c .
c
 , where , where  .
d .
d
 , where M = number of reflections, N = number of parameters refined. , where M = number of reflections, N = number of parameters refined.
|
|
| Empirical formula | C14H20N5 |
| M | 258.35 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a/Å | 7.17860(10) |
| b/Å | 17.8246(3) |
| c/Å | 12.0348(2) |
| β/° | 105.6190(10) |
| V/Å3 | 1483.06(4) |
| Z | 4 |
| T/K | 135(2) |
| D c/g cm−3 | 1.157 |
| μ/cm−1 | 0.73 |
| λ(MoKα)/Å | 0.71073 |
| Max. transmission coefficient | 0.9956 |
| Min. tramsmission coefficient | 0.9437 |
| Reflections collected | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 213 213 |
| Unique reflections | 3935 (Rint = 0.0361) |
| Reflections observed | 3028 |
| R indexb [I > 2σ(I)] | R 1 = 0.0828 |
| R indicesb (all data) | R 1 = 0.1073 |
| wR 2 = 0.1868 | |
| Weighting coefficientsc | a = 0.0391 |
| b = 1.5207 | |
| Goodness-of-fitd on F2 | 1.168 |
Spectroscopic studies
Infrared spectra were taken using a Bio-Rad FTS-40 FTIR spectrophotometer with ±1 cm−1 resolution, and scanned in the range 400–4000 cm−1. UV-Vis spectroscopy was carried out on a Hewlett Packard 8452A Diode Array Spectrophotometer, scanning from 190 to 820 nm. Samples were prepared as KBr pressed pellets (ca. 5% w/w) for both experiments.Results and discussion
The reaction of [Et4N]I and TCNE leads to the formation of [Et4N]2[TCNE]2, eqn. (1),3[Et4N]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3I− 3I−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2TCNE 2TCNE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) → →![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Et4N]I3 [Et4N]I3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Et4N]2[TCNE]2 [Et4N]2[TCNE]2 | (1) |
![Atom labeling and thermal ellipsoid (40%) plot of [Et4N]2[TCNE]2. H atoms and disordered alkyl chains are omitted for clarity. Key bond distances (Å) and angles (°) are: C(1)–C(2) 1.418(3), C(1)–C(2)′ 2.827(3), C(1)–C(3) 1.424(3), C(1)–C(4) 1.418(3), C(2)–C(5) 1.425(3), C(2)–C(6) 1.432(3), C(3)–N(3) 1.149(3), C(4)–N(4) 1.145(3), C(5)–N(5) 1.146(3), C(6)–N(6) 1.150(3); C(1)–C(2)–C(1)′ 90.2(2), C(2)–C(1)–C(2)′
89.8(2), C(3)–C(1)–C(4) 118.5(2), C(5)–C(2)–C(6) 117.3(2), C(1)–C(2)–C(5) 120.7(2), C(1)–C(2)–C(6) 120.7(2), C(2)–C(1)–C(3) 120.3(2), C(2)–C(1)–C(4) 119.9(2); C(4)–C(1)–C(2)–C(6) 169.0(2), C(3)–C(1)–C(2)–C(5) 164.6(2).](/image/article/2001/CE/b108455k/b108455k-f1.gif) | ||
| Fig. 1 Atom labeling and thermal ellipsoid (40%) plot of [Et4N]2[TCNE]2. H atoms and disordered alkyl chains are omitted for clarity. Key bond distances (Å) and angles (°) are: C(1)–C(2) 1.418(3), C(1)–C(2)′ 2.827(3), C(1)–C(3) 1.424(3), C(1)–C(4) 1.418(3), C(2)–C(5) 1.425(3), C(2)–C(6) 1.432(3), C(3)–N(3) 1.149(3), C(4)–N(4) 1.145(3), C(5)–N(5) 1.146(3), C(6)–N(6) 1.150(3); C(1)–C(2)–C(1)′ 90.2(2), C(2)–C(1)–C(2)′ 89.8(2), C(3)–C(1)–C(4) 118.5(2), C(5)–C(2)–C(6) 117.3(2), C(1)–C(2)–C(5) 120.7(2), C(1)–C(2)–C(6) 120.7(2), C(2)–C(1)–C(3) 120.3(2), C(2)–C(1)–C(4) 119.9(2); C(4)–C(1)–C(2)–C(6) 169.0(2), C(3)–C(1)–C(2)–C(5) 164.6(2). | ||
![Intra- and inter-dimer [TCNE]22− interactions in [Et4N]2[TCNE]2. Click image or here to access a 3D representation.](/image/article/2001/CE/b108455k/b108455k-f2.gif) | ||
| Fig. 2 Intra- and inter-dimer [TCNE]22− interactions in [Et4N]2[TCNE]2. Click image or 2.htm to access a 3D representation. | ||
[TCNE]22− is a π dimer with a two-electron four-centered bond between the [TCNE]− monomer moieties as reported earlier.3 The intradimer CC bond distance is 2.827(3) Å, comparable to those reported that range from 2.833 to 3.09 Å.3 Another manifestation of the intradimer CC bonding is the change in hybridization of the central carbons, which leads to the trans-NC–C–C–CN angle increasing from 0° for planar [TCNE]− to 6.6° for [Et4N]2[TCNE]2. This value is in accord with those reported that range from 3.6 to 6.5°.3
Dimerization of [TCNE]− leads to overlap of the b2g singly occupied molecular orbital (SOMO) on each moiety to form bonding and antibonding orbitals of b2u and b1g symmetry (Fig. 3), respectively, with a 1A1g (b2u2b1g0) ground state electronic structure for the dimer.
![Schematic diagram of the b2g SOMO [TCNE]− orbitals overlapping to form bonding and antibonding orbitals of b2u and b1g symmetry, respectively, for the [TCNE]22− dimer.](/image/article/2001/CE/b108455k/b108455k-f3.gif) | ||
| Fig. 3 Schematic diagram of the b2g SOMO [TCNE]− orbitals overlapping to form bonding and antibonding orbitals of b2u and b1g symmetry, respectively, for the [TCNE]22− dimer. | ||
Intradimer [TCNE]22− bond formation also leads to a change in the IR spectrum, which differs with respect to its fragments, i.e.
[TCNE]˙−. For the single anion moiety, νC![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) N IR absorptions occur at 2183 and 2144 cm−1,10 and the νCC absorption is inactive and not observed. In contrast, the as reported π-[TCNE]22− exhibits three νC
N IR absorptions occur at 2183 and 2144 cm−1,10 and the νCC absorption is inactive and not observed. In contrast, the as reported π-[TCNE]22− exhibits three νC![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) N vibrations, at 2191 ± 2 (m), 2172 ± 2 (s), and 2161 ± 2 (s) cm−1, and νC
N vibrations, at 2191 ± 2 (m), 2172 ± 2 (s), and 2161 ± 2 (s) cm−1, and νC![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) C at 1360 (s) cm−1.3 The solid state spectrum of [Et4N]2[TCNE]2
(Fig. 4) shows that new IR bands, arising from the intradimer bonding, occur at 2191 (m), 2170 (s), 2163 (s)
(νC
C at 1360 (s) cm−1.3 The solid state spectrum of [Et4N]2[TCNE]2
(Fig. 4) shows that new IR bands, arising from the intradimer bonding, occur at 2191 (m), 2170 (s), 2163 (s)
(νC![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) N), and 1365 (s) cm−1
(νC
N), and 1365 (s) cm−1
(νC![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) C). The ca. 1360 cm−1 absorption is due to the antisymmetric combination of the intrafragment CC stretches of each fragment's central CC bond, which becomes allowed and gains intensity due to electron-vibrational coupling.11
C). The ca. 1360 cm−1 absorption is due to the antisymmetric combination of the intrafragment CC stretches of each fragment's central CC bond, which becomes allowed and gains intensity due to electron-vibrational coupling.11
![Solid state IR spectrum of [TCNE]22− in [Et4N]2[TCNE]2.](/image/article/2001/CE/b108455k/b108455k-f4.gif) | ||
| Fig. 4 Solid state IR spectrum of [TCNE]22− in [Et4N]2[TCNE]2. | ||
The electronic absorption spectrum of [TCNE]˙− has an absorption at 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375 cm−1
(428 nm; 2.89 eV) in solution, which has 17 vibrational overtones.10 This 2B2u → 2B3g transition broadens in the solid state (Fig. 5). [TCNE]22− exhibits this broad absorption at 26
375 cm−1
(428 nm; 2.89 eV) in solution, which has 17 vibrational overtones.10 This 2B2u → 2B3g transition broadens in the solid state (Fig. 5). [TCNE]22− exhibits this broad absorption at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 cm−1
(382 nm; 3.24 eV) as well as a new absorption at 16
150 cm−1
(382 nm; 3.24 eV) as well as a new absorption at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 cm−1
(593 nm; 2.09 eV)
(Fig. 5). The latter absorption is assigned to the b2u2b1g0
(1A1g) → b2u1b1g1
(1B1u)
transition and gives these dimeric compounds the observed dark blue–purple color. This value is higher in energy than the 15
850 cm−1
(593 nm; 2.09 eV)
(Fig. 5). The latter absorption is assigned to the b2u2b1g0
(1A1g) → b2u1b1g1
(1B1u)
transition and gives these dimeric compounds the observed dark blue–purple color. This value is higher in energy than the 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 cm−1
(654 nm, 1.90 eV) reported for Tl2[TCNE]2, [(Me2N)2CC(NMe2)2][TCNE]2, and CrI(C6H6)]2[TCNE]2.3
300 cm−1
(654 nm, 1.90 eV) reported for Tl2[TCNE]2, [(Me2N)2CC(NMe2)2][TCNE]2, and CrI(C6H6)]2[TCNE]2.3
Intradimer bond formation also affects the magnetic properties. When doublet S = 1/2 [TCNE]− fragments approach each other to form the π-[TCNE]22− dimer, Fig. 3, the spins interact to form an S = 0 singlet ground state and an S = 1 triplet excited state (or vice versa). Strong antiferromagnetic coupling between the spins leads to the singlet ground state. However, upon warming, thermal population of the triplet excited state may be achieved, as has frequently been reported.12 As noted for related [TCNE]22− dimers,3 diamagnetic-like behavior is observed for [Et4N]2[TCNE]2 due to population of only the singlet state at and below room temperature.
Acknowledgements
The authors (J. S. M. and R. E. D. S.) gratefully acknowledge the support from the NSF (Grant No. CHE9320478) and the DOE (Grant No. DE FG 03-93ER45504) and discussions with Professor Gordon T. Yee (Virginia Polytechnic Institute) and Professor Juan J. Novoa (University of Barcelona). R. D. S. gratefully acknowledges the support from the Petroleum Research Foundation (Grant No. 34614-AC3).Notes and references
- G. Kaupp and J. Boy, Angew. Chem., Int. Ed. Engl., 1997, 36, 48 CrossRef CAS.
- F. Toda, Eur. J. Org. Chem., 2000, 1377 CrossRef CAS.
- J. J. Novoa, P. Lafuente, R. E. Del Sesto and J. S. Miller, Angew. Chem., Int. Ed., 2001, 40, 2540 CrossRef CAS.
- M. T. Johnson, C. F. Campana, B. M. Foxman, W. Desmarais, M. J. Vela and J. S. Miller, Eur. J. Chem., 2000, 6, 1805 Search PubMed.
- H. Bock, K. Ruppert, D. Fenske and H. Goesmann, Z. Anorg. Allg. Chem., 1995, 595, 275 CrossRef.
- J. S. Miller, D. M. O'Hare, A. Charkraborty and A. J. Epstein, J. Am. Chem. Soc., 1989, 111, 7853 CrossRef CAS.
- D. A. Lemervoskii, R. A. Stukan, B. N. Tarasevich, Tu. L. Slovokhotov, M. Yu. Antipin, A. E. Kalinin and Yu. T. Struchov, Struct. Khim., 1981, 7, 240 Search PubMed.
- J. R. Fox, B. M. Foxman, D. Guerrer, J. S. Miller and A. H. Reis Jr., J. Mater. Chem., 1996, 6, 1627 RSC.
- Calculated from one-half of the average trans-NC–C–C–CN dihedral angles using CrystalMaker5: D. C. Palmer, CrystalMaker, Interactive Crystallography for MacOS, CrystalMaker Software, Oxford, 2001.
- D. L. Jeanmarie, M. R. Suchanski and R. P. Van Duyne, J. Am. Chem. Soc., 1975, 97, 1699 CrossRef; D. A. Dixon and J. S. Miller, J. Am. Chem. Soc., 1987, 109, 3656 CrossRef CAS.
- Similar results were noted for π-[TCNQ]22−: M. J. Rice, N. O. Lipari and S. Strassler, Phys. Rev. Lett., 1977, 39, 1359 Search PubMed.
- See, for example: B. Bleaney and K. D. Bowers, Proc. R. Soc. London, Ser. A, 1952, 214, 451 Search PubMed; K. M. Chi, J. C. Calabrese and J. S. Miller, Mol. Cryst. Liq. Cryst., 1989, 176, 173 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |

![Solid state UV-Vis spectrum of [TCNE]22− in [Et4N]2[TCNE]2.](/image/article/2001/CE/b108455k/b108455k-f5.gif)