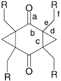
Packing of symmetrical 1,3,5,7-tetrasubstituted tricyclo[5.1.0.03,5]octane-2,6-diones
D. S.
Yufit
*a,
S. I.
Kozhushkov
b,
J. A. K.
Howard
a and
A.
de Mejere
b
aDepartment of Chemistry, University of Durham, Durham, UK DH1 3LE. E-mail: d.s.yufit@durham.ac.uk
bInstitut für Organische Chemie der Georg-August-Universität, Göttingen, D 370077, Germany
Abstract
Crystal structures of four symmetrical 1,3,5,7-tetrasubstituted tricyclo[5.1.0.03,5]octane-2,6-diones have been determined and their packing is discussed. In the absence of strong intermolecular interactions, the methylene groups of the cyclopropane rings form C–H⋯O weak hydrogen bonds which determine the packing of the molecules within the crystal. In the case of syn-isomers, unusual multi-centred interactions have been found.
Introduction
One of the most fascinating synthetic problems in the chemistry of strained polyspirocyclopropane systems (triangulanes) is the preparation of cyclo[6]triangulane1 [so-called davidane (1)] – the first potentially stable member of a family of still elusive cyclic triangulanes. Exploring the possible synthetic approaches to this compound we have prepared several symmetrical 1,3,5,7-tetrasubstituted tricyclo[5.1.0.03,5]octane-2,6-diones (2–5) and determined their crystal structures. Most of the few previously known 2,6-dioxotricyclo[5.1.0.03,5]octanes are 4,8-disubstituted derivatives2 and no crystal structures of symmetrically substituted compounds have been described. Compounds 2 and 3 possess an anti-configuration of the two cyclopropane rings in the tricyclooctane skeleton; compounds 4 and 5 are syn-isomers. Crystals of 4 have been obtained as a benzene solvate.The gradual change of substituents and configuration in this series of compounds provides an opportunity to analyse the influence of these alterations upon the packing of the molecules within the crystals. The molecules 3–5, which do not have groups, form strong hydrogen bonds which are particularly interesting. Here, the weak intermolecular interactions should determine the packing of the molecules, and in structures 3–5 a number of possible interactions (C–H⋯O, Cl⋯Cl, C–H⋯Cl, etc.) may be formed. The role of different types of such interactions in structural chemistry is now well established and various aspects of the chemistry of weak hydrogen bonds have been summarised recently by Desiraju and Steiner.3 In this communication we report the results of single-crystal X-ray structural analyses of 2–5 and discuss the packing of these molecules within the crystals.
Results and discussion
The molecular structures of 2–5 are shown in Fig. 1, and the main bond lengths are given in Table 1; the parameters of the intermolecular contacts, discussed below, are listed in Table 3. | ||
| Fig. 1 Molecules 2–5 and labelling schemes. Thermal ellipsoids are given at the 50% probability level. Click each image to access its 3D representation. | ||
|
|
||||
|---|---|---|---|---|
| Bond | 2 | 3 | 4 | 5 |
| a All bond lengths are given in Å. | ||||
| a | 1.222(1) | 1.217(3) | 1.220(2) | 1.218(5) |
| 1.214(2) | 1.219(5) | |||
| b | 1.497(2) | 1.497(3) | 1.497(2) | 1.507(3) |
| 1.502(2) | 1.501(3) | 1.511(2) | 1.507(3) | |
| c | 1.535(2) | 1.538(3) | 1.553(2) | 1.541(4) |
| d | 1.521(2) | 1.523(3) | 1.517(2) | 1.522(4) |
| 1.525(2) | 1.525(3) | 1.509(2) | 1.520(4) | |
| e | 1.516(2) | 1.503(3) | 1.511(2) | 1.509(4) |
| 1.523(2) | 1.505(3) | 1.503(2) | 1.506(4) | |
| f | 1.429(2) | 1.806(3) | 1.807(2) | 1.469(4) |
| 1.434(2) | 1.808(3) | 1.799(2) | 1.473(4) | |
In spite of the apparent similarity, molecules 2–5 have different molecular symmetry: the approximate symmetry of the anti-molecules 2 and 3 is C2h, whereas for syn-4 and -5 it is Cs and C2v, respectively. Not surprisingly, these symmetrical molecules occupy special positions within the crystals. Molecules 2 and 3 lie across centres of symmetry, whereas 4 and 5, being syn-isomers, are on mirror planes which pass through both carbonyl bonds of the molecules. The central six-membered rings in the anti-isomers 2 and 3 are planar. Within the syn-molecules 4 and 5 these rings are non-planar and the bending of this ring within molecule 4 is asymmetric because of the different orientations of the substituents [bending angles along C(2)⋯C(2a) and C(3)⋯C(3a) lines are 22.4 and 9.9° in 4 and 15.7 and 16.3° in 5]. The configurational differences (syn/anti) of molecules 2–5 do not affect the bond lengths in their tricyclooctane systems, which are remarkably similar in the studied molecules (except for the slight asymmetry in 3, see Table 1). Moreover, the dihedral angles between the plane of four sp3 carbon atoms of the six-membered ring and cyclopropane planes are almost identical, regardless of the conformation of the molecules, and they vary from 76.7° in 4 to 78.9° in 3. However, the packing of these molecules within the crystals is quite different.
The pair of intra-molecular hydrogen bonds O(3)–H⋯O(2) (Fig. 1, Table 2) fixes the relative orientation of the terminal hydroxy groups within molecule 2. The hydroxy groups O(2)–H take part as a donor in intermolecular H-bonds, which link molecules in layers perpendicular to the (101) direction [Fig. 2(a)]. These strong hydrogen bonds are responsible for the main ‘pattern’ of the arrangement of the molecules within the crystals. It is worth mentioning that the planes of the central six-membered rings of molecules in structure 2 are approximately parallel to each other. The layers are held together by weaker hydrogen bonds C(4)–H(41)⋯O(1) between the cyclopropane CH2 group and the carbonyl oxygen atom [Fig. 2(b)]. It has been reported,4 that H atoms of three-membered rings form weak hydrogen bonds more readily than those of ‘ordinary’ CH2 groups, and the parameters of this bond within the crystal of 2 correspond well to those described in ref. 4.
 | ||
| Fig. 2 (a) Packing of the molecules 2 (H atoms of methylene groups are omitted); (b) C–H⋯O interactions in structure 2. | ||
| D–H⋯A | d(D–H)/Å | d(H⋯A)/Å | d(D⋯A)/Å | ∠(DHA)/° |
|---|---|---|---|---|
| a Intra-molecular hydrogen bond. b Multi-centred contact. | ||||
| 2 | ||||
| O(3)–H(3O)⋯O(2) (−x⊕+⊕2, −y⊕+⊕2 , −z⊕+⊕1)a | 0.86(2) | 1.92(2) | 2.7146(14) | 154(2) |
| O(2)–H(2O)⋯O(3) (x⊕−⊕1/2, −y⊕+⊕3/2, z⊕−⊕1/2) | 0.82(2) | 1.90(2) | 2.7082(14) | 169(2) |
| C(4)–H(41)⋯O(1) (x⊕−⊕1/2, −y⊕+⊕3/2, z⊕+⊕1/2) | 0.96(2) | 2.46(2) | 3.382(2) | 161(1) |
| 3 | ||||
| C(5)–Cl(1)⋯Cl(2) (x, −0.5⊕−⊕y, 0.5⊕+⊕z) | — | 3.575(1) | — | 87.8(1) |
| C(6)–Cl(2)⋯Cl(1) (x, −0.5⊕−⊕y, −0.5⊕+⊕z) | — | 3.575(1) | — | 156.7(1) |
| C(4)–H(41)⋯O(1) (x, 1⊕+⊕y, z) | 0.92(3) | 2.75(3) | 3.146(3) | 107(2) |
| C(4)–H(41)⋯O(1) (−x, 0.5⊕+⊕y, 1.5⊕−⊕z) | 0.92(3) | 2.79(2) | 2.942(3) | 90(2) |
| C(6)–H(61)⋯Cl(1) (−x, −y, 1⊕−⊕z) | 0.96(3) | 2.84(3) | 3.779(3) | 166(2) |
| 4 | ||||
| C(5)–H(52)⋯O(1) (0.5⊕+⊕x, y, 0.5⊕−⊕z)b | 0.98(2) | 2.46(2) | 3.214(2) | 134(1) |
| C(10)–H(10)⋯O(2) (−1⊕+⊕x, y, z) | 0.96(3) | 2.70(4) | 3.626(3) | 161(3) |
| C(5)–H(51)⋯Cl(1) (1.5⊕−⊕x, −y, −0.5⊕+⊕z) | 0.95(2) | 2.89(2) | 3.571(1) | 130(1) |
| C(6)–H(62)⋯Cl(1) (1⊕−⊕x, −y, 1⊕−⊕z) | 0.96(2) | 2.83(2) | 3.550(2) | 132(2) |
| C(7)–H(71)⋯Cl(2) (1.5⊕−⊕x, −y, 0.5⊕+⊕z) | 0.94(3) | 2.95(3) | 3.778(2) | 147(2) |
| 5 | ||||
| C(5)–H(51)⋯O(1) (x, y, 1⊕+⊕z)b | 0.92(3) | 2.64(2) | 3.327(4) | 135(3) |
| C(5)–H(51)⋯O(2) (x, 1⊕+⊕y, z)b | 0.92(3) | 2.54(3) | 3.323(4) | 144(3) |
| C(5)–H(52)⋯O(5) (0.5⊕−⊕x, 1⊕−⊕y, 0.5⊕+⊕z) | 0.96(3) | 2.33(3) | 3.281(4) | 168(3) |
The packing of molecules 2 is easily understandable. This is not always the case, however, for compounds 3–5 where no strong hydrogen bonds could be formed and, therefore, interactions of other types become dominant.
Molecule 3 was obtained from 2 by replacement of the OH groups with chlorine atoms, and in the absence of strong hydrogen donors within structure 3, weak interactions play the major role in the packing of the molecules. In this case the Cl⋯Cl interactions are dominant. The geometry of the contacts is very similar to that found within the structure of molecular chlorine5 where the Cl–Cl bond is perpendicular to that of the next molecule, although in chlorine itself the Cl⋯Cl distances are shorter (3.284 Å). This geometry belongs to type II (according to previous classification6) and in this configuration the concentration of charge in the valence shell at one of the atoms is directed towards the depletion of charge in the other. Indeed, within the structure of 3 the C(6)–Cl(2) bond is directed towards the Cl(1) atom of the adjacent molecule [C–Cl(2)⋯Cl(1) 156.7°] and is perpendicular to the corresponding Cl(1)–C bond [Cl(2)⋯Cl(1)–C 87.8°]. These interactions arrange the molecules in layers perpendicular to (100) [Fig. 3(a)], where CH2Cl groups are concentrated in the interlayer areas and the hydrocarbon frameworks are within the layers [Fig. 3(b)]. However, the ‘perpendicular’ geometry of Cl⋯Cl interactions makes any parallel orientation of the six-membered rings of adjacent molecules within the layers unfavourable and the molecules are ‘perpendicular’ to each other. There are also close (C⋯O 2.943 Å) C–H⋯O contacts between molecules in the layer, but the geometry of these contacts (C–H⋯O 90.1°) does not allow us to regard them as C–H⋯O hydrogen bonds. The shortest interlayer contacts are of the C–H⋯Cl type. The discussion about the nature of such interactions is still ongoing in the literature (see, for example, ref. 7 and references therein) and is beyond the scope of this paper. However, in this particular case the geometrical parameters of these contacts satisfy the criteria for weak hydrogen bonds.
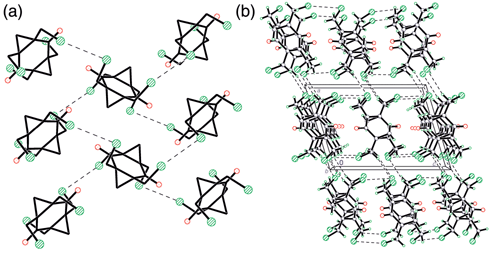 | ||
| Fig. 3 (a) Cl⋯Cl interactions in the layers of molecules 3 within the crystal (H atoms are omitted); (b) crystal packing of 3 viewed along the b-axis. | ||
The reversion of configuration of the two three-membered rings as in syn-4 brings the cyclopropane rings into close proximity and results in a dramatic change of packing. The ‘central’ hydrogen atoms of the methylene groups form unusual double C(5)–H(51)⋯O(1) intermolecular contacts with the carbonyl atom of the adjacent molecule [Fig. 4(a)]. At the same time, Cl⋯Cl contacts disappear altogether and the shortest intermolecular distance between halogen atoms exceeds 4 Å. This probably implies that these double C–H⋯O interactions are much more favourable than those of the Cl⋯Cl type. These contacts link the molecules in chains along the a-direction. The shortest (and in this case, probably, van der Waals) contacts between chains are of the C–H⋯Cl type. The chains form channels, where solvate benzene molecules are located [Fig. 4(b)]. There are no very close contacts between benzene molecules inside the channels or between the benzene and ‘host’ molecules. The shortest contact is for C(10)–H(10)⋯O(2) with the ‘free’ carbonyl oxygen atom of the host.
 | ||
| Fig. 4 (a) Double C–H⋯O interactions between the host molecules in structure 4 (H atoms of the CH2Cl groups are omitted); (b) packing of the molecules in the crystal of 4 (viewed along the a-axis). | ||
In the case of molecule 5 all cyclopropyl hydrogen atoms take part in C–H⋯O contacts with adjacent molecules, but by a different, even more unusual manner than in 4. The H(51) atom forms almost symmetrical, multi-centred hydrogen bonds with the carbonyl oxygen atoms, and in turn each of these oxygen atoms acts as an acceptor for two C–H⋯O bonds [Fig. 5(a)]. These bonds link molecules in sheets perpendicular to the (011) direction. Atom H(52) takes part in a more conventional C–H⋯O interaction, but with the oxygen atom O(5) of the S![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O double bond. Geometrical parameters of this bond suggest that this is the strongest interaction among similar bonds in structures 2–5.
O double bond. Geometrical parameters of this bond suggest that this is the strongest interaction among similar bonds in structures 2–5.
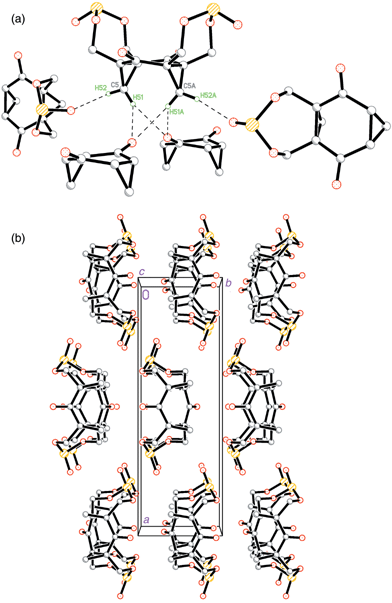 | ||
| Fig. 5 (a) Multi-centred C–H⋯O interactions in structure 5 (parts of the ‘acceptor’ molecules are omitted for clarity); (b) packing of the molecules within crystal 5. | ||
Experimental
The general strategy of the synthesis of 2–5 has been described in ref. 1, and the detailed synthetic procedure will be published elsewhere. All data were collected on a Bruker SMART CCD 1 K diffractometer [λ(MoKα) radiation, graphite monochromator] equipped with an Oxford Cryostream low-temperature device. Details of the data collection and refinement are given in Table 3. No absorption correction was applied. The unit cells of crystals 4 and 5 are close to tetragonal ones; however, attempts to apply higher symmetry resulted in unreasonably high Rint values (>0.3) and problems with the structure solutions and refinement, etc., and were abandoned. All structures were solved by direct methods and refined by full-matrix, least squares on F2. All non-hydrogen atoms were refined anisotropically; all hydrogen atoms were located on the difference Fourier maps and refined isotropically.| Compound | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| a Click b108369d.txt for full crystallographic data (CCDC 171043–171046). | ||||
| Empirical formula | C12H16O6 | C12H12Cl4O2 | C18H18Cl4O2 | C12H12O8S2 |
| Crystal dimensions/mm | 0.22⊕×⊕0.20⊕×⊕0.18 | 0.52⊕×⊕0.14⊕×⊕0.02 | 0.40⊕×⊕0.15⊕×⊕0.10 | 0.12⊕×⊕0.09⊕×⊕0.02 |
| Crystal system | Monoclinic | Monoclinic | Orthorhombic | Orthorhombic |
| Space group | P21/n | P21/c | Pnma | Pmn21 |
| M | 256.25 | 330.02 | 408.12 | 348.34 |
| a/Å | 7.3226(2) | 9.0855(5) | 12.0462(4) | 18.4528(7) |
| b/Å | 8.4959(2) | 6.1050(3) | 12.1220(3) | 6.0455(2) |
| c/Å | 9.2703(3) | 12.3553(6) | 12.8780(4) | 6.0486(2) |
| β/° | 92.97(2) | 106.51(1) | 90 | 90 |
| V/Å3 | 575.95(3) | 657.04(6) | 1880.5(1) | 674.76(4) |
| Z | 2 | 2 | 4 | 2 |
| T/K | 120.0(2) | 120.0(2) | 120.0(2) | 100.0(2) |
| D c/Mg m−3 | 1.478 | 1.668 | 1.442 | 1.714 |
| μ/mm−1 | 0.119 | 0.890 | 0.637 | 0.436 |
| λ/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| F(000) | 272 | 336 | 840 | 360 |
| θ Range for data collection/° | 3.25–30.28 | 2.34–30.34 | 2.31–30.30 | 1.10–30.37 |
| Index ranges | −6⊕≤⊕h⊕≤⊕10 | −12⊕≤⊕h⊕≤⊕10 | −16⊕≤⊕h⊕≤⊕15 | −25⊕≤⊕h⊕≤⊕25 |
| −11⊕≤⊕k⊕≤⊕11 | −7⊕≤⊕k⊕≤⊕8 | −16⊕≤⊕k⊕≤⊕16 | −8⊕≤⊕k⊕≤⊕8 | |
| −13⊕≤⊕l⊕≤⊕12 | −17⊕≤⊕l⊕≤⊕16 | −18⊕≤⊕l⊕≤⊕17 | −8⊕≤⊕l⊕≤⊕8 | |
| Reflections collected | 5224 | 6195 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356 356 |
7864 |
| Independent reflections | 1603 | 1823 | 2820 | 2124 |
| R int | 0.048 | 0.076 | 0.051 | 0.067 |
| Completeness to max θ/% | 93.5 | 92.1 | 96.0 | 96.2 |
| Data, restraints, parameters | 1603, 0, 114 | 1823, 0, 106 | 2820, 0, 166 | 2124, 1, 131 |
| Goodness-of-fit on F2 | 1.086 | 1.026 | 1.057 | 1.046 |
| Final R indices [I⊕>⊕2σ(I)] | R 1⊕=⊕0.0419 | R 1⊕=⊕0.0485 | R 1⊕=⊕0.0363 | R 1⊕=⊕0.0478, |
| wR 2⊕=⊕0.1056 | wR 2⊕=⊕0.0873 | wR 2⊕=⊕0.0856 | wR 2⊕=⊕0.1035 | |
| R indices (all data) | R 1⊕=⊕0.0530 | R 1⊕=⊕0.0771 | R 1⊕=⊕0.0475 | R 1⊕=⊕0.0650 |
| wR 2⊕=⊕0.1130 | wR 2⊕=⊕0.0961 | wR 2⊕=⊕0.0915 | wR 2⊕=⊕0.1123 | |
| Largest difference, peak and hole/e Å−3 | 0.381, −0.219 | 0.436, −0.404 | 0.407, −0.327 | 0.555, −0.509 |
Conclusions
Analysis of the packing of molecules 2–5 in the crystals shows that the methylene groups of the three-membered rings play an important role in the arrangement of molecules in the absence of strong hydrogen bonds. In the case of syn-isomers, unusual, previously unknown strong ‘double’ C–H⋯O interactions have been observed. The non-cyclopropane methylene groups are relatively inactive in forming intermolecular interactions within the structures studied.Acknowledgements
D. S. Y. thanks the EPSRC (UK) for financial support.References
- A. de Meijere and S. I. Kozhushkov, Chem. Rev., 2000, 100, 93 CrossRef CAS.
- S. Tsuboi, H. Furutani, A. Takeda, K. Kawazoe and S. Sato, Bull. Chem. Soc. Jpn., 1987, 60, 2475 CAS; J. Heller, A. S. Dreiding, R. Grieb and A. Niggli, Angew. Chem., Int. Ed., 1972, 11, 236 CrossRef CAS; V. A. Mamedov, E. A. Berdnikov, I. A. Litvinov and L. G. Kus'mina, Izv. Akad. Nauk. SSSR, Ser. Khim., 1995, 1294 Search PubMed.
- G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond in Structural Chemistry and Biology, Oxford University Press, Oxford, 1999 Search PubMed.
- F. H. Allen, J. P. M. Lommerse, V. J. Hoy, J. A. K. Howard and G. R. Desiraju, Acta Crystallogr., Sect. B, 1996, 52, 734 CrossRef.
- V. G. Tsirelson, P. F. Zou, T. H. Tang and R. F. W. Bader, Acta Crystallogr., Sect. A, 1995, 51, 143 CrossRef.
- V. R. Pedireddi, D. S. Reddy, B. S. Goud, D. C. Craig, A. D. Rae and G. R. Desiraju, J. Chem. Soc., Perkin Trans. 2, 1994, 2353 RSC.
- P. K. Thallapally and A. Nangia, CrystEngComm, 2001, 27 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |