The first AgI-bis(sulfinyl) coordination polymer forming a unique macrometallacyclic lamellar network
Xian-He
Bu
*,
Wei
Chen
,
Miao
Du
and
Ruo-Hua
Zhang
Department of Chemistry, Nankai University, Tianjin, 300071, P. R. China. E-mail: buxh@nankai.edu.cn
Abstract
The first AgI complex with a flexible meso-bis(sulfinyl) ligand forming a unique two-dimensional, 36-membered macrometallacyclic molecular square array is reported.
Introduction
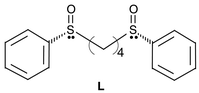
Crystal engineering and construction of coordination networks with fascinating structural topologies has attracted great attention in recent years due to the potential of these compounds as functional materials.1 Concurrent with this has been the development of multi-dimensional networks based primarily upon linking metal centers with rigid bridging components, such as 4,4′-bipyridine, etc.2 Far less common has been the use of flexible bridging units in the construction of extended networks,3,4 which are attractive because the flexibility and conformation freedoms of such ligands can offer the possibility for the construction of unprecedented frameworks with useful properties.Herein is reported the first AgI complex with a flexible meso-bis(sulfinyl) ligand (L) forming a unique macrometallacyclic lamellar network.
The underlying principle of this study design is as follows. Bis(sulfinyl) compounds have advantages that: (a) they are bifunctional ligands in terms that both S and O atoms are potential donor atoms and they have ditopic coordination sites which may allow structures with higher dimensions; (b) each S atom with a pyramidal geometry is an asymmetric center which may introduce acentric sites into the resulting crystals [for example, if ligands with an RS configuration were used (as in this study), the resulting network as a whole would be mesomeric; however, if ligands with RR or SS configurations were used, one would expect to obtain chiral networks with acentric cavities]; (c) such structurally flexible ligands have the possibility of forming diverse extended networks which might be deeply influenced and controlled by the length of the spacer unit and the template anions; and (d) the bis(sulfinyl) ligands having ditopic donor sites (S and O), of which the chemistry is still less developed, are likely to be widely relevant in the context of a vast variety of metal moieties and may be expected to develop into a rich coordination chemistry.
Experimental
General procedures and instructions
Most of the starting materials and solvents for the syntheses were obtained commercially and purified by standard procedures prior to use. FT-IR spectra were recorded on a Nicolet Magna-IR 750 spectrometer in the range 4000–400 cm−1 using KBr pellets. Elemental analyses were obtained using an Elementar Vario EL analyzer.Synthesis
L was prepared according to the reported procedures.5 Complex 1, [AgL2·ClO4]∞, was obtained by mixing equimolar amounts of L dissolved in CHCl3 and AgClO4 in acetone at room temperature. The colorless single crystals were obtained by slow evaporation of the solvent in the dark (yield 40%). 1 is light-sensitive and should be kept in the dark. Anal. calc. for C32H36AgClO8S4: C, 46.86; H, 4.42. Found: C, 46.68; H, 4.69%. IR (KBr pellet, cm−1): 1089s, 1037s, 749m, 690m.Caution! While we experienced no problems in handling perchlorates in this study, these should be handled with great care due to the potential for explosion.
Crystallographic data collection and structure determination
X-Ray measurement was performed on a Bruker Smart 1000 CCD X-ray diffractometer equipped with graphite monochromatized MoKα radiation. The structure of complex 1 was solved by direct method using the SHELXS-97 program.6a SADABS6b absorption corrections were also applied. The refinement was done by a full-matrix, least squares method based on F2.6 (For a summary of the crystal data for 1 see Table 1.)| Parameter | 1 |
|---|---|
| a Click b105297g.txt for full crystallographic data (CCDC 164457). | |
| Formula | Ag(C16H18O2S2)2ClO4 |
| Crystal dimensions/mm | 0.35⊕×⊕0.35⊕×⊕0.40 |
| M | 820.17 |
| Crystal system | Tetragonal |
| Space group | P4/ncc |
| a/Å | 15.189(2) |
| b/Å | 15.189(2) |
| c/Å | 15.191(3) |
| V/Å3 | 3504.7(10) |
| Z | 4 |
| T/K | 293⊕±⊕2 |
| D c/g cm−3 | 1.554 |
| μ/mm−1 | 0.938 |
| F(000) | 1680 |
| Measured reflections | 13321 |
| Unique reflections | 1560 |
| R (Rint) | 0.0317 (0.0566) |
| R w | 0.0824 |
| Goodness of fit on F2 | 1.001 |
| Largest difference, peak and hole/e Å−3 | 0.479, −0.264 |
Results and discussion
In the IR spectra of 1, the absorption bands at 1036 and 996 cm−1 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) are lower than the corresponding S
O stretching) are lower than the corresponding S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations in the metal-free ligand (1040 cm−1), indicating that the L ligands are bound to AgI centers through the oxygen atoms of sulfoxide moieties.7 The existence of perchlorate anions in 1 was confirmed by IR spectra with the presence of two strong bands at 1085 and 624 cm−1. Satisfactory elemental analysis was obtained for all of the compounds.
O vibrations in the metal-free ligand (1040 cm−1), indicating that the L ligands are bound to AgI centers through the oxygen atoms of sulfoxide moieties.7 The existence of perchlorate anions in 1 was confirmed by IR spectra with the presence of two strong bands at 1085 and 624 cm−1. Satisfactory elemental analysis was obtained for all of the compounds.
As shown in Fig. 1, the AgI centers of 1 are coordinated by four oxygen atoms of the sulfoxide moieties of four distinct L ligands and form a perfect square-planar coordination geometry, with all four Ag–O bonds being equivalent [2.318(2) Å] and the adjacent O–Ag–O angles being almost 90° [89.985(2)°] (Table 2).
 | ||
| Fig. 1 ORTEP view of the coordination geometry around each AgI center of complex 1. Click image or 1.htm to access a 3D representation. | ||
| Ag(1)–O(1) | 2.318(2) | S(1)–O(1) | 1.503(2) |
| O(1A)–Ag(1)–O(1C) | 179.17(11) | O(1A)–Ag(1)–O(1) | 89.985(2) |
| S(1)–O(1)–Ag(1) | 119.67(12) | O(1)–S(1)–C(11) | 107.98(13) |
| C(11)–S(1)–C(1) | 96.29(13) |
This is a very rare example of the coordination geometry of AgI.8 Normally, S atoms have a higher affinity than O atoms with the soft AgI ions.9 However, in complex 1 it is the O atoms, and not the S atoms, of the ditopic ligands that coordinate to the AgI ions, probably due to the steric hindrance of the bulky phenyl groups connected to the S atoms.7 Two of the four sulfur atoms bound to the oxygens are R- whereas the other two have the S-configuration. Therefore, the network of 1 as a whole is mesomeric but with local acentric sites in the cavities. The adjacent AgI centers are linked viaL in orthogonal directions, which results in a molecular domain constituted by a highly symmetrical 36-membered Ag4L4 macrocycle with the adjacent Ag–Ag distances of 11.177 Å. Two AgI corners in the diagonal position fold toward the upside or downside to form a ‘valley-like’ molecular unit, which has S4 symmetry (Fig. 2). The perchlorate ions filling in the cavity of the ‘valley’ [side view of the network, Fig. 3(b)] may act as templates for the formation of the network and consequently keep the structure stable [Fig. 2(b)]. Adjacent rings are fused in a unique two-dimensional lamellar structure [Fig. 3(a), the top view of the network of 1, a (4,4) net].
 | ||
| Fig. 2 (a) The basket-like 36-membered Ag4L4 repeating unit with S4 symmetry of 1. Ag(1)–O(1) 2.318(2) Å, O(1A)–Ag(1)–O(1B) 89.985(2)°. (b) Space-filling diagram showing the accommodation of a perchlorate anion in the basket-like repeating unit from the top view. Ag purple; S yellow; Cl green; O red; C grey. | ||
 | ||
| Fig. 3 (a) View from the c axis showing the two-dimensional square-grid of 1. Hydrogen atoms are omitted for clarity. (b) View of the wave-like layer stacking of 1 in the c direction including the perchlorate anions. | ||
To the best of our knowledge, complex 1 is the first structurally characterized AgI complex with a flexible bis(sulfinyl) ligand, in which each AgI center takes a rare, perfect, square coordination geometry, and where the network as a whole is mesomeric because L has RS configuration. The ‘valley’ sheet of 1 is a unique structural motif.
In conclusion, a novel AgI complex forming unique macrometallacyclic lamellar networks has been reported. A systematic study on bis(sulfinyl) ligands by varying the spacer units of their backbone, and further studies using bis(sulfinyl) ligands with SS or RR configurations for the construction of chiral networks, are currently underway in our laboratory.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 29971019) and the Trans-Century Talents Training Program Foundation from the State Education Ministry of China.References
- For example, see: G. B. Gardner, D. Venkataraman, J. S. Moore and S. Lee, Nature, 1995, 374, 792 CrossRef CAS; H. O. Stumpt, L. Ouahab, Y. Pei, D. Grandjean and O. Kahn, Science, 1993, 261, 447 CrossRef PubMed; O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703 CrossRef; A. J. Blake, N. R. Champness, P. Hubberstey, W. S. Li, M. A. Withersby and M. Schröder, Coord. Chem. Rev., 1999, 183, 117 CrossRef; M. Munakata, L. P. Wu, M. Yamamoto, T. Kuroda-Sowa and M. Maekawa, J. Am. Chem. Soc., 1996, 118, 3117 CrossRef; X. H. Bu, K. Biradha, T. Yamaguchi, M. Nishimura, T. Ito, K. Tanaka and M. Shionoya, Chem. Commun., 2000, 1953 RSC.
- For example, see: S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1460 CrossRef CAS and references therein; M. Fujita, M. Aoyagi and K. Ogura, Bull. Chem. Soc. Jpn., 1998, 71, 1799 CrossRef and references therein; C. V. K. Sharma, G. A. Broker, J. G. Huddleston, J. W. Baldwin, R. M. Metzger and R. D. Rogers, J. Am. Chem. Soc., 1999, 121, 1137 CrossRef; K. Biradha, C. Seward and M. J. Zaworotko, Angew. Chem., 1999, 111, 584 CrossRef; K. Biradha, C. Seward and M. J. Zaworotko, Angew. Chem., Int. Ed., 1999, 38, 492 CrossRef.
- For example, see: D. M. L. Goodgame, D. A. Grachvogel, I. Hussain, A. J. P. White and D. J. Williams, Inorg. Chem., 1999, 38, 2057 CrossRef CAS PubMed and references therein; K. W. Kim and M. G. Kanatzidis, J. Am. Chem. Soc., 1992, 114, 4878 CrossRef.
- For example, see: G. K. H. Shimizu, G. D. Enright, C. I. Ratcliffe, J. A. Ripmeester and D. D. M. Wayner, Angew. Chem., 1998, 110, 1510 CrossRef CAS; G. K. H. Shimizu, G. D. Enright, C. I. Ratcliffe, J. A. Ripmeester and D. D. M. Wayner, Angew. Chem., Int. Ed., 1998, 37, 1407 CrossRef; M. Hong, W. Su, R. Cao, M. Fujita and J. Lu, Chem. Eur. J., 2000, 6, 427 CrossRef.
- R. H. Zhang, Y. L. Zhan and J. T. Chen, Synth. React. Inorg. Met.-Org. Chem., 1995, 25, 293 CrossRef CAS; R. H. Zhang, B. Q. Ma, X. H. Bu, H. G. Wang and X. K. Yao, Polyhedron, 1997, 16, 1123 CrossRef.
- (a) G. M. Sheldrick, SHELXS-97, Program for X-Ray Crystal Structure Solution, University of Göttingen, Germany, 1997 Search PubMed; G. M. Sheldrick, SHELXL-97, Program for X-Ray Crystal Structure Refinement, University of Göttingen, Germany, 1997 Search PubMed; (b) G. M. Sheldrick, SADABS, Siemens Area Detector Absorption Correction Program, University of Göttingen, Germany, 1994 Search PubMed.
- H. B. Kagan and B. Ronan, Rev. Heteroat. Chem., 1992, 7, 92 Search PubMed.
- B. Lippert and D. Neugebauer, Inorg. Chim. Acta, 1980, 46, 171 Search PubMed.
- S. G. Murray and F. R. Hartley, Chem. Rev., 1981, 81, 365 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |