DOI:
10.1039/B105080J
(Paper)
CrystEngComm, 2001,
3, 155-158
Construction of a 3D array of cadmium(II) using squarate as a building block
Received
11th June 2001
, Accepted 16th August 2001
Abstract
A unique three-dimensional coordination polymer of cadmium(II), [Cd(C4O4)(H2O)2]n1
(C4O42−⊕=⊕squarate dianion), has been synthesized and characterized by single crystal structural analysis. The molecular structure shows that each bridging squarate dianion functions as a four-fold monodentate ligand to form a cage-like channel network. The compound crystallizes in the rhombohedral system, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148) with a⊕=⊕b⊕=⊕11.765(6) Å and c⊕=⊕14.920(7) Å, γ⊕=⊕120°, Z⊕=⊕9, V⊕=⊕1788.5(15) Å3.
Thermal investigation reveals that the dehydrated compound is remarkably stable.
(no. 148) with a⊕=⊕b⊕=⊕11.765(6) Å and c⊕=⊕14.920(7) Å, γ⊕=⊕120°, Z⊕=⊕9, V⊕=⊕1788.5(15) Å3.
Thermal investigation reveals that the dehydrated compound is remarkably stable.
Introduction
Assembling extended structure compounds by selecting organic spacers (e.g. 4,4′-bipyridine, pyrazine, dicarboxylic acids, etc.) and inorganic metal ion species of suitable chemical structure and coordination geometry, respectively, may yield a series of novel networks with various sizes and shapes of void space or cavity.1 Such types of materials are being investigated intensively because of their multi-dimensional applications in the field of catalysis,2a nonlinear optics,2b electrical conductivity,2c molecular recognition2d and molecular sieves.2e Among the different organic spacers, the squarate dianion (C4O42−) is an interesting cyclic compound with aromaticity and
it can function as a bridging ligand with possible μ-2, μ-3 and μ-4 between metal ions3
(Scheme 1). The most fully investigated metal squarate is M(C4O4)(H2O)4
[M⊕=⊕Mn(II), Co(II), Ni(II), Fe(II), Zn(II) and Cu(II)]4 with two bridging C4O42−
(μ-2) ligands at trans positions, which form an infinite chain structure. Each metal ion is also bonded with four water molecules to form a distorted octahedral geometry. West and Niu5 first reported another type of metal squarate with the formula M(C4O4)(H2O)2
[M(II)⊕=⊕Fe(II),
Co(II), Ni(II) and Zn(II)]. Later three-dimensional cubic structures were found to have the formula M(C4O4)(H2O)2
[M⊕=⊕Ni(II)6 and Fe(II)4b], M(C4O4)(H2O)2(MeCO2H·H2O)1/3
[M⊕=⊕Zn(II), Ni(II) and Mn(II)]7 and Co(C4O4)(H2O)2·0.33H2O4b, where C4O42− functions as a bridging ligand between four metal ions (μ-4).
Besides these two types of metal squarate, another type with the formula M(HC4O4)2(H2O)4
[M⊕=⊕Fe(II)4b and Mn(II)8] has been found, which is a two-dimensional sheet-like network built from (HC4O4)2 and M(H2O)4 units.
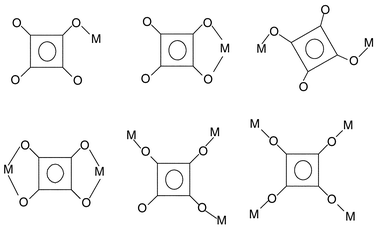 |
| | Scheme 1
Binding modes of the squarate dianion.
| |
In the course of our studies on polymeric coordination compounds9 here we have chosen cadmium(II) as the metal ion due to its d10 configuration, which permits a wide variety of geometries and coordination numbers, with the squarate dianion as an effective building block in order to synthesize an uncharged polymeric network. Here, we report a bridging μ-4 squarate coordination polymer of cadmium(II), exhibiting a 3D channel network, and solid state thermal studies, which show that the cadmium squarate framework is remarkably stable after dehydration.
Experimental
High purity 3,4-dihydroxy-3-cyclobutene-1,2-dione (squaric acid) was purchased from the Aldrich Chemical Company Inc. and used as-received. All other chemicals used were of AR grade.
Physical measurements
Elemental analyses (carbon and hydrogen) were performed using a Perkin-Elmer 240C elemental analyser, and cadmium(II) contents were estimated gravimetrically. Infrared spectra (4000–400 cm−1) were taken using a Nicolet Magna-IR 750 spectrometer, series-II, where KBr was used as the dispersal medium. Thermal analysis (TGA–DTA) was carried out on a Shimadzu DT-30 thermal analyzer under dinitrogen (flow rate: 30 cm3 min−1). The sample particle size was within 150–200 mesh. X-Ray powder diffraction data were collected using a Seifert XRD-3000P instrument, using Cu-Kα
(λ⊕=⊕1.5406 Å) radiation (30 kV/30 mA); the primary slits were 3 mm/soller/2 mm and the secondary slits were soller/0.1 mm.
Preparation of [Cd(C4O4)(H2O)2]n1
An aqueous solution (5 cm3) of squaric acid (1 mmol, 0.114 g) was slowly added to an aqueous solution (10 cm3) of Cd(MeCO2)2·2H2O (1 mmol, 0.246 g). Immediately, a white crystalline solid of complex 1 separated out, and this was washed with methanol and dried in a vacuum desiccator. It was dissolved in water at ca. 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and the solution was stored in a CaCl2 desiccator. Suitable single crystals were separated out after a few days (80% yield). Anal. calc. for C4H4O6Cd: C, 18.43; H, 1.53; Cd, 43.16. Found: C, 18.42; H, 1.54; Cd, 43.12%.
°C and the solution was stored in a CaCl2 desiccator. Suitable single crystals were separated out after a few days (80% yield). Anal. calc. for C4H4O6Cd: C, 18.43; H, 1.53; Cd, 43.16. Found: C, 18.42; H, 1.54; Cd, 43.12%.
Crystallographic data collection and refinement
Reflection data were measured using an AFC7S diffractometer in ω–2θ scan mode using graphite monochromated Mo-Kα radiation. The intensity data were corrected for Lorentz and polarization effects10 and an empirical absorption correction based on Ψ scan was also employed. A total of 4561 reflections was measured, of which 1441 were unique (Rint⊕=⊕0.117). The structure was solved by Patterson synthesis and followed by Fourier methods. Complex neutral atom scattering factors11 were used throughout. All calculations were carried out using SHELXS-86,12 SHELXL-97,13 PLATON9914 and ORTEP-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 programs. Details of data collection and refinement are given in Table 1.
15 programs. Details of data collection and refinement are given in Table 1.
Table 1
Crystal data and structure refinement for [Cd(C4O4)(H2O)2]n1a
| Parameter |
|
Click b105080j.txt for full crystallographic data (CCDC 155487).
|
| Empirical formula |
C4H4CdO6 |
| Crystal dimensions/mm |
0.20⊕×⊕0.20⊕×⊕0.20 |
|
M
|
260.48 |
| Crystal system |
Trigonal (rhombohedral) |
| Space group |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148)
(no. 148) |
|
a/Å |
11.765(6) |
|
b/Å |
11.765(6) |
|
c/Å |
14.920(7) |
|
α/° |
90 |
|
β/° |
90 |
|
γ/° |
120 |
|
V/Å3 |
1788.5(15) |
|
Z
|
9 |
|
T/K |
293(2) |
|
D
c/Mg m−3 |
2.177 |
|
λ(Mo-Kα)/Å |
0.71073 |
|
μ(Mo-Kα)/mm−1 |
2.729 |
|
F(000) |
1116 |
|
θ Range/° |
2.4–32.5 |
| Total data |
4561 |
| Unique data |
1441 (Rint⊕=⊕0.117) |
| Observed data [I⊕>⊕2σ(I)] |
1435 |
|
R
1
|
0.0419 |
|
wR
2, S |
0.1046, 0.90 |
Results and discussion
The IR spectrum of compound 1 (not shown) reveals a strong and broad absorption in the region 1490–1530 cm−1, which is assigned to a mixture of C–C and C–O stretching vibrations of the C4O42− ligand. In addition, only one sharp band at ca. 1761 cm−1, assigned to C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O, indicates that all four oxygen atoms of C4O42− are in the same environment.
O, indicates that all four oxygen atoms of C4O42− are in the same environment.
Structural description of complex 1
X-Ray structure determination reveals that the polymer has the stoichiometry Cd(C4O4)(H2O)2, having a 3D infinite network. An ORTEP-3 view with atom numbering scheme is shown in Fig. 1 in which every C4O42− moiety binds to four different cadmium(II) centres, i.e. each C4O42− functions as a four-fold monodentate bridging ligand. Thus the binding geometry of C4O42− corresponds very well to a system of completely delocalized π-electrons.
![ORTEP-3 plot of a portion of the 3D polymeric complex [Cd(C4O4)(H2O)2]n with atom labelling scheme. Thermal ellipsoids are drawn at the 50% probability level. (Colour code: Cd blue, O red, C black, H green.) (Symmetry codes: a⊕=⊕1/3⊕−⊕x⊕+⊕y, 2/3⊕−⊕x, −1/3⊕+⊕z; b⊕=⊕1/3⊕+⊕x, 2/3⊕+⊕y, −1/3⊕+⊕z; c⊕=⊕1⊕−⊕x⊕+⊕y, 1⊕−⊕x, z; d⊕=⊕1/3⊕−⊕y, −1/3⊕+⊕x⊕−⊕y, −1/3⊕+⊕z;
e⊕=⊕1/3⊕+⊕x, −1/3⊕+⊕y, −1/3⊕+⊕z; f⊕=⊕1⊕−⊕y, x⊕−⊕y, z; g⊕=⊕2/3⊕−⊕x⊕+⊕y, 1/3⊕−⊕x, 1/3⊕+⊕z; h⊕=⊕−1/3⊕+⊕x, −2/3⊕+⊕y, 1/3⊕+⊕z; i⊕=⊕−x⊕+⊕y, −x, z; j⊕=⊕2/3⊕−⊕y, 1/3⊕+⊕x⊕−⊕y, 1/3⊕+⊕z; k⊕=⊕2/3⊕+⊕x, 1/3⊕+⊕y, 1/3⊕+⊕z;
l⊕=⊕−y, x⊕−⊕y, z; m⊕=⊕2/3⊕−⊕x, 1/3⊕−⊕y, 1/3⊕−⊕z; n⊕=⊕−1/3⊕+⊕y, 1/3⊕−⊕x⊕+⊕y, 1/3⊕−⊕z; p⊕=⊕1⊕−⊕x, 1⊕−⊕y, −z). Click image or here to access a 3D representation.](/image/article/2001/CE/b105080j/b105080j-f1.gif) |
| | Fig. 1
ORTEP-3 plot of a portion of the 3D polymeric complex [Cd(C4O4)(H2O)2]n with atom labelling scheme. Thermal ellipsoids are drawn at the 50% probability level. (Colour code: Cd blue, O red, C black, H green.) (Symmetry codes: a⊕=⊕1/3⊕−⊕x⊕+⊕y, 2/3⊕−⊕x, −1/3⊕+⊕z; b⊕=⊕1/3⊕+⊕x, 2/3⊕+⊕y, −1/3⊕+⊕z; c⊕=⊕1⊕−⊕x⊕+⊕y, 1⊕−⊕x, z; d⊕=⊕1/3⊕−⊕y, −1/3⊕+⊕x⊕−⊕y, −1/3⊕+⊕z;
e⊕=⊕1/3⊕+⊕x, −1/3⊕+⊕y, −1/3⊕+⊕z; f⊕=⊕1⊕−⊕y, x⊕−⊕y, z; g⊕=⊕2/3⊕−⊕x⊕+⊕y, 1/3⊕−⊕x, 1/3⊕+⊕z; h⊕=⊕−1/3⊕+⊕x, −2/3⊕+⊕y, 1/3⊕+⊕z; i⊕=⊕−x⊕+⊕y, −x, z; j⊕=⊕2/3⊕−⊕y, 1/3⊕+⊕x⊕−⊕y, 1/3⊕+⊕z; k⊕=⊕2/3⊕+⊕x, 1/3⊕+⊕y, 1/3⊕+⊕z;
l⊕=⊕−y, x⊕−⊕y, z; m⊕=⊕2/3⊕−⊕x, 1/3⊕−⊕y, 1/3⊕−⊕z; n⊕=⊕−1/3⊕+⊕y, 1/3⊕−⊕x⊕+⊕y, 1/3⊕−⊕z; p⊕=⊕1⊕−⊕x, 1⊕−⊕y, −z). Click image or 1.htm to access a 3D representation.
| |
In the asymmetric unit, each cadmium(II) centre is attached to four different C4O42− moieties and two water molecules to form an octahedron with the CdO6 chromophore. Each cadmiun atom occupies an inversion centre. The two oxygen atoms of water molecules (O1W, O1Wm, m⊕=⊕2/3⊕−⊕x, 1/3⊕−⊕y, 1/3⊕−⊕z) and other two oxygen atoms (O1, O1m) from the two squarato moieties form the equatorial plane, and the oxygen atoms (O2n and O2f, n⊕=⊕−1/3⊕+⊕y, 1/3⊕−⊕x⊕+⊕y, 1/3⊕−⊕z; f⊕=⊕1⊕−⊕y, x⊕−⊕y, z) of another two squarato
moieties occupy the trans axial positions (selected bond length and angle data are given in Table 2).
Table 2
Selected bond lengths (Å) and angles (°) for [Cd(C4O4)(H2O)2]n1
| Symmetry codes: m⊕=⊕2/3⊕−⊕x, 1/3⊕−⊕y, 1/3⊕−⊕z; n⊕=⊕−1/3⊕+⊕y, 1/3⊕−⊕x⊕+⊕y, 1/3⊕−⊕z; f⊕=⊕1⊕−⊕y, x⊕−⊕y, z. |
| Cd–O1 |
2.276(3) |
Cd–O2 |
2.311(2) |
| Cd–O1W |
2.234(4) |
O1–C1 |
1.253(4) |
| C1–C2 |
1.460(4) |
O2–C2 |
1.253(5) |
| |
| O1–Cd–O1W |
95.19(12) |
O1–Cd–O2f |
93.25(12) |
| O1–Cd–O1m |
180.00 |
O1–Cd–O1Wm |
84.82(12) |
| O1–Cd–O2n |
86.75(12) |
O1W–Cd–O2f |
83.21(12) |
| O1m–Cd–O1W |
84.82(12) |
O1W–Cd–O1Wm |
180.00 |
| O1W–Cd–O2n |
96.80(13) |
O1m–Cd–O2f |
86.74(12) |
| O1Wm–Cd–O2f |
96.77(12) |
O2f–Cd–O2n |
180.00 |
| O1m–Cd–O1Wm |
95.17(12) |
O1m–Cd–O2n |
93.26(12) |
| O1Wm–Cd–O2n |
83.21(13) |
|
|
It should be noted that each cadmium(II) sits with equatorial atoms without any deviation from the mean plane. There are four branches emanating from every cadmium(II) centre, and in each branch each cadmium(II) is connected to another cadmium(II) centre via a C4O42− bridge, which in turn produces three other branches forming a robust 3D polymeric network (Fig. 2).
![View of the polymeric array formed by [Cd(C4O4)(H2O)2]n. (Colour code: Cd blue, O red, C black, H green.)](/image/article/2001/CE/b105080j/b105080j-f2.gif) |
| | Fig. 2
View of the polymeric array formed by [Cd(C4O4)(H2O)2]n. (Colour code: Cd blue, O red, C black, H green.)
| |
A void calculation using the PLATON99 software shows that the network contains a channel of 386.5 Å3 per unit cell (21.6%) when viewed down the c-axis (CPK model, Fig. 3). Formation of this 3D network may be due to the unique bridging mode of the squarate dianion. The Cd–O bond distances are in the range 2.234(3)–2.311(2) Å. The water molecules form relatively strong O–H⋯O hydrogen bonds (O⋯O 2.76 and 2.74 Å; O–H⋯O 176 and 154°) with squarato oxygens (Table 3).
![CPK view of [Cd(C4O4)(H2O)2]n along the c-axis illustrating the channels. (Colour code: Cd blue, O red, C black, H green.)](/image/article/2001/CE/b105080j/b105080j-f3.gif) |
| | Fig. 3
CPK view of [Cd(C4O4)(H2O)2]n along the c-axis illustrating the channels. (Colour code: Cd blue, O red, C black, H green.)
| |
Table 3
Hydrogen bond lengths (Å) and angles (°) for [Cd(C4O4)(H2O)2]n1
| D–H⋯A |
D–H |
H⋯A |
D⋯A |
D–H⋯A |
| Symmetry codes: n⊕=⊕−1/3⊕+⊕y, 1/3⊕−⊕x⊕+⊕y, 1/3⊕−⊕z; p⊕=⊕1⊕−⊕x, 1⊕−⊕y, −z. |
| O1W–H2⋯O1n |
0.91(6) |
1.90(5) |
2.744(5) |
154(5) |
| O1W–H1⋯O2p |
0.92(4) |
1.84(4) |
2.759(4) |
176(4) |
Thermal properties
The multidentate functionality of C4O42− and its ability to generate such tightly held bulky building units have a significant role on the stability of this material, while the possibility of the elimination of water molecules presents an opportunity for achieving a porous material with coordinatively unsaturated cadmium(II) centres. Thermogravimetric analysis (TGA) on this material shows a stepwise loss of two water molecules at ca. 220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, corroborating the presence of strong H-bonding interactions for the Osquarate⋯H–O–H moiety (see Fig. 1). The dehydrated compound remains stable up to 365
°C, corroborating the presence of strong H-bonding interactions for the Osquarate⋯H–O–H moiety (see Fig. 1). The dehydrated compound remains stable up to 365![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C without any weight loss, indicating a robust cadmium squarate framework. Interestingly, we have observed that the dehydrated compound becomes rehydrated in the presence of water. Comparison of the X-ray powder diffraction pattern
of the starting material [T⊕=⊕298 K; data collection range (2θ)⊕=⊕10–40°] with that of the dehydrated solid shows that the diffraction lines in the latter remain almost similar but only different positions and line widths. This may indicate a slight deformation of the pore structure due to the empty space created by loss of coordinated water molecules. It is worth noting that the XRD pattern of the rehydrated compound shows coincidence of the peak positions and intensities with those observed for the original solid (Fig. 4).
°C without any weight loss, indicating a robust cadmium squarate framework. Interestingly, we have observed that the dehydrated compound becomes rehydrated in the presence of water. Comparison of the X-ray powder diffraction pattern
of the starting material [T⊕=⊕298 K; data collection range (2θ)⊕=⊕10–40°] with that of the dehydrated solid shows that the diffraction lines in the latter remain almost similar but only different positions and line widths. This may indicate a slight deformation of the pore structure due to the empty space created by loss of coordinated water molecules. It is worth noting that the XRD pattern of the rehydrated compound shows coincidence of the peak positions and intensities with those observed for the original solid (Fig. 4).
![XRD patterns of the (a) as-synthesized material [Cd(C4O4)(H2O)2]n, (b) dehydrated solid [Cd(C4O4)]n, and (c) rehydrated solid resulting from the reintroduction of water molecules into the coordination sphere of the dehydrated solid.](/image/article/2001/CE/b105080j/b105080j-f4.gif) |
| | Fig. 4
XRD patterns of the (a) as-synthesized material [Cd(C4O4)(H2O)2]n, (b) dehydrated solid [Cd(C4O4)]n, and (c) rehydrated solid resulting from the reintroduction of water molecules into the coordination sphere of the dehydrated solid.
| |
This indicates that the internal structure of the material remains intact and that heating and water removal do not cause much deformation in the Cd–squarate–Cd framework. The proposed dehydrated structure (CPK model, Fig. 5) is predicted to have a channel volume of 486.2 Å3 per unit cell (27.2%). The thermal and structural stability of the dehydrated solid and the presence of the coordinated unsaturated metal centres may enable the material to act as a solvent inclusion compound.
![Perspective CPK view of the proposed structure of the dehydrated complex [Cd(C4O4)]n along the c-axis illustrating the channels. (Colour code: Cd blue, O red, C black).](/image/article/2001/CE/b105080j/b105080j-f5.gif) |
| | Fig. 5
Perspective CPK view of the proposed structure of the dehydrated complex [Cd(C4O4)]n along the c-axis illustrating the channels. (Colour code: Cd blue, O red, C black).
| |
Acknowledgements
The authors wish to thank the Council of Scientific and Industrial Research, New Delhi, for financial support (granted to N. R. C.).
References
- O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703 CrossRef CAS; D. Venkataraman, G. B. Garden, S. Lee and T. S. Moore, J. Am. Chem. Soc., 1995, 117, 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 CrossRef; S. Subramanian and M. J. Zaworotko, Angew. Chem., Int. Ed. Engl., 1994, 35, 2127 Search PubMed; D. Braga, A. Angeloni, L. Maini, A. W. Götz and F. Grepioni, New J. Chem., 1999, 17 RSC.
600 CrossRef; S. Subramanian and M. J. Zaworotko, Angew. Chem., Int. Ed. Engl., 1994, 35, 2127 Search PubMed; D. Braga, A. Angeloni, L. Maini, A. W. Götz and F. Grepioni, New J. Chem., 1999, 17 RSC.
-
(a) K. Maruoka, N. Murase and H. Yamamoto, J. Org. Chem., 1993, 58, 2938 CrossRef CAS;
(b) C. Chen and K. S. Suslick, Coord. Chem. Rev., 1993, 128, 293 CrossRef CAS;
(c) B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546 CrossRef CAS;
(d) M. Kondo, T. Yoshitomi, K. Seki, H. Matsuzaka and S. Kitagawa, Angew. Chem., Int. Ed. Engl., 1997, 36, 1725 CrossRef CAS;
(e) A. Yuan, J. Zou, B. Li, Z. Zha, C. Duan, Y. Liu, Z. Xu and S. Keizer, Chem. Commun., 2000, 1279 Search PubMed.
- X. Solans, M. Aguilo, A. Gleizes, J. Faus, M. Julve and M. Verdaguer, Inorg. Chem., 1990, 29, 775 CrossRef CAS; J. A. C. Vanooijen, J. Reedijk and A. L. Spek, Inorg. Chem., 1979, 18, 1184 CrossRef; M. Y. Khan, Y. Chang, Q. Chen, J. Salta, Y. Lee, C. J. O'Connor and J. Zubieta, Inorg. Chem., 1994, 33, 6340 CrossRef; R. Soules, F. Dahan, J. P. Laurent and P. Castan, J. Chem. Soc., Dalton Trans., 1988, 587 RSC.
-
(a) A. Weiss, E. Riegler, I. Alt, H. Böhme and C. Robl, Z. Naturforsch., B, 1986, 41, 18 Search PubMed;
(b) C. R. Lee, C. C. Wang and Y. Wang, Acta Crystallogr., Sect. B, 1996, 52, 966 CrossRef.
- R. West and H. Y. Niu, J. Am. Chem. Soc., 1963, 85, 2589 CrossRef CAS.
- M. Habenschuss and B. C. Gerstein, J. Chem. Phys., 1974, 61, 854 CrossRef.
- A. Weiss, E. Riegler and C. Robl, Z. Naturforsch., B, 1986, 41, 1329 Search PubMed; A. Weiss, E. Riegler and C. Robl, Z. Naturforsch., B, 1986, 41, 1333 Search PubMed.
- O. M. Yaghi, G. Li and T. L. Groy, J. Chem. Soc., Dalton Trans., 1995, 727 RSC.
- A. Mondal, G. Mostafa, A. Ghosh, I. R. Laskar and N. Ray Chaudhuri, J. Chem. Soc., Dalton Trans., 1999, 9 RSC; A. Mondal, G. Mostafa, A. Ghosh and N. Ray Chaudhuri, J. Chem. Res. (S), 1998, 570 RSC.
- A. C. T. North, D. C. Philips and F. S. Mathews, Acta Crystallogr. Sect. A, 1968, 24, 351 CrossRef.
-
International Tables for Crystallography, ed. A. J. C. Wilson, Kluwer Academic Publishers, Dordrecht, The Netherlands, 1992,
Vol. C, Tables 4.2.6.8 and 6.1.1.4 Search PubMed.
-
G. M. Sheldrick, SHELXS-86, Program for the Solution of Crystal Structures, University of Göttingen, Germany, 1986.
-
G. M. Sheldrick, SHELXL-97, Program for Crystal Structure Determination, University of Göttingen, Germany, 1997.
-
A. L. Spek, PLATON99, Molecular Geometry Program, University of Utrecht, The Netherlands, 1999.
- ORTEP-3: L. J. Farrugia, J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2001 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148) with a⊕=⊕b⊕=⊕11.765(6) Å and c⊕=⊕14.920(7) Å, γ⊕=⊕120°, Z⊕=⊕9, V⊕=⊕1788.5(15) Å3.
Thermal investigation reveals that the dehydrated compound is remarkably stable.
(no. 148) with a⊕=⊕b⊕=⊕11.765(6) Å and c⊕=⊕14.920(7) Å, γ⊕=⊕120°, Z⊕=⊕9, V⊕=⊕1788.5(15) Å3.
Thermal investigation reveals that the dehydrated compound is remarkably stable.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and the solution was stored in a CaCl2 desiccator. Suitable single crystals were separated out after a few days (80% yield). Anal. calc. for C4H4O6Cd: C, 18.43; H, 1.53; Cd, 43.16. Found: C, 18.42; H, 1.54; Cd, 43.12%.
°C and the solution was stored in a CaCl2 desiccator. Suitable single crystals were separated out after a few days (80% yield). Anal. calc. for C4H4O6Cd: C, 18.43; H, 1.53; Cd, 43.16. Found: C, 18.42; H, 1.54; Cd, 43.12%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 programs. Details of data collection and refinement are given in Table 1.
15 programs. Details of data collection and refinement are given in Table 1.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148)
(no. 148)![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O, indicates that all four oxygen atoms of C4O42− are in the same environment.
O, indicates that all four oxygen atoms of C4O42− are in the same environment.
![ORTEP-3 plot of a portion of the 3D polymeric complex [Cd(C4O4)(H2O)2]n with atom labelling scheme. Thermal ellipsoids are drawn at the 50% probability level. (Colour code: Cd blue, O red, C black, H green.) (Symmetry codes: a⊕=⊕1/3⊕−⊕x⊕+⊕y, 2/3⊕−⊕x, −1/3⊕+⊕z; b⊕=⊕1/3⊕+⊕x, 2/3⊕+⊕y, −1/3⊕+⊕z; c⊕=⊕1⊕−⊕x⊕+⊕y, 1⊕−⊕x, z; d⊕=⊕1/3⊕−⊕y, −1/3⊕+⊕x⊕−⊕y, −1/3⊕+⊕z;
e⊕=⊕1/3⊕+⊕x, −1/3⊕+⊕y, −1/3⊕+⊕z; f⊕=⊕1⊕−⊕y, x⊕−⊕y, z; g⊕=⊕2/3⊕−⊕x⊕+⊕y, 1/3⊕−⊕x, 1/3⊕+⊕z; h⊕=⊕−1/3⊕+⊕x, −2/3⊕+⊕y, 1/3⊕+⊕z; i⊕=⊕−x⊕+⊕y, −x, z; j⊕=⊕2/3⊕−⊕y, 1/3⊕+⊕x⊕−⊕y, 1/3⊕+⊕z; k⊕=⊕2/3⊕+⊕x, 1/3⊕+⊕y, 1/3⊕+⊕z;
l⊕=⊕−y, x⊕−⊕y, z; m⊕=⊕2/3⊕−⊕x, 1/3⊕−⊕y, 1/3⊕−⊕z; n⊕=⊕−1/3⊕+⊕y, 1/3⊕−⊕x⊕+⊕y, 1/3⊕−⊕z; p⊕=⊕1⊕−⊕x, 1⊕−⊕y, −z). Click image or here to access a 3D representation.](/image/article/2001/CE/b105080j/b105080j-f1.gif)
![View of the polymeric array formed by [Cd(C4O4)(H2O)2]n. (Colour code: Cd blue, O red, C black, H green.)](/image/article/2001/CE/b105080j/b105080j-f2.gif)
![CPK view of [Cd(C4O4)(H2O)2]n along the c-axis illustrating the channels. (Colour code: Cd blue, O red, C black, H green.)](/image/article/2001/CE/b105080j/b105080j-f3.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, corroborating the presence of strong H-bonding interactions for the Osquarate⋯H–O–H moiety (see Fig. 1). The dehydrated compound remains stable up to 365
°C, corroborating the presence of strong H-bonding interactions for the Osquarate⋯H–O–H moiety (see Fig. 1). The dehydrated compound remains stable up to 365![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C without any weight loss, indicating a robust cadmium squarate framework. Interestingly, we have observed that the dehydrated compound becomes rehydrated in the presence of water. Comparison of the X-ray powder diffraction pattern
of the starting material [T⊕=⊕298 K; data collection range (2θ)⊕=⊕10–40°] with that of the dehydrated solid shows that the diffraction lines in the latter remain almost similar but only different positions and line widths. This may indicate a slight deformation of the pore structure due to the empty space created by loss of coordinated water molecules. It is worth noting that the XRD pattern of the rehydrated compound shows coincidence of the peak positions and intensities with those observed for the original solid (Fig. 4).
°C without any weight loss, indicating a robust cadmium squarate framework. Interestingly, we have observed that the dehydrated compound becomes rehydrated in the presence of water. Comparison of the X-ray powder diffraction pattern
of the starting material [T⊕=⊕298 K; data collection range (2θ)⊕=⊕10–40°] with that of the dehydrated solid shows that the diffraction lines in the latter remain almost similar but only different positions and line widths. This may indicate a slight deformation of the pore structure due to the empty space created by loss of coordinated water molecules. It is worth noting that the XRD pattern of the rehydrated compound shows coincidence of the peak positions and intensities with those observed for the original solid (Fig. 4).
![XRD patterns of the (a) as-synthesized material [Cd(C4O4)(H2O)2]n, (b) dehydrated solid [Cd(C4O4)]n, and (c) rehydrated solid resulting from the reintroduction of water molecules into the coordination sphere of the dehydrated solid.](/image/article/2001/CE/b105080j/b105080j-f4.gif)
![Perspective CPK view of the proposed structure of the dehydrated complex [Cd(C4O4)]n along the c-axis illustrating the channels. (Colour code: Cd blue, O red, C black).](/image/article/2001/CE/b105080j/b105080j-f5.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 CrossRef; S. Subramanian and M. J. Zaworotko, Angew. Chem., Int. Ed. Engl., 1994, 35, 2127 Search PubMed; D. Braga, A. Angeloni, L. Maini, A. W. Götz and F. Grepioni, New J. Chem., 1999, 17 RSC.
600 CrossRef; S. Subramanian and M. J. Zaworotko, Angew. Chem., Int. Ed. Engl., 1994, 35, 2127 Search PubMed; D. Braga, A. Angeloni, L. Maini, A. W. Götz and F. Grepioni, New J. Chem., 1999, 17 RSC.