Crystal engineering of a three-dimensional coordination polymer based on both covalent and O–H⋯O hydrogen bonding interactions of bifunctional ligands
Jack Y.
Lu
* and
Vaughn
Schauss
Department of Chemistry, University of Houston-Clear Lake, Houston, Texas 77058, USA. E-mail: lu@cl.uh.edu
Abstract
A three-dimensional coordination polymer, [(H2O)2Cu(pdc)] 1 (pdc⊕=⊕pyridine-3,5-dicarboxylate), has been rationally designed and synthesized under hydrothermal reaction conditions. The structure is self-assembled from bifunctional pyridinecarboxylates and water molecules. Carboxylate groups in 1 contribute to both covalent and hydrogen bonds. The structure is stabilized by offset aryl–aryl face–face packing interactions.
Introduction
Recently, researchers have undertaken a number of approaches towards the design and synthesis of metal–organic coordination polymers based upon the concept of crystal engineering.1–4 Introduced to the design of more efficient topochemical reactions in the early 1960s by Rabinowich and Schmidt,5 the concept of crystal engineering is now widely used in organic supramolecular chemistry and metal–organic coordination polymers for achieving physical and chemical targets. In 1989, the first extended metal–organic polymeric network deliberately designed and synthesized was reported.6 Intense research activities in this field have recently produced many new metal–organic polymers of great diversity.1–4In recent years, the self-assembly of extended one-, two- and three-dimensional metal–organic network polymers via hydrogen bonding has attracted much attention.7,8 The utilization of hydrogen bonding is a well known design principle in the construction of supramolecular architecture and crystal engineering.9–11 Our interests in exploring new coordination networks focus on the bifunctional organic ligands. Bifunctional ligands have the advantages of both sturdy covalent bonding and flexible, non-covalent bonding interactions and solubility, such as hydrogen bonds. Hydrogen bond-containing coordination polymers may have potential applications in the biomedical field and nanomaterials.12 Pyridinecarboxylate ligands have shown interesting properties in the construction of coordination polymers.13,14 The introduction of hydrogen bonds can be achieved by the use of coordinating water molecules: these coordinating water molecules tend to form hydrogen bonds with neighboring nitrogen- and oxygen-containing organic units in order to link the network structures.12a,15 For example, pyridine-3,5-dicarboxylate (pdc) is a good candidate for this purpose. One of the possible networks from pdc coordination to metal ions (Scheme 1) is to form a two-dimensional sheet.
 | ||
| Scheme 1 | ||
Under hydrothermal conditions the open-metal center in Scheme 1 can easily adopt water molecules at the axial positions to result in trigonal bipyramidal coordination metal centers. The coordination water molecules will probably interact with the carboxylate groups in adjacent layers via hydrogen bonds to give a three-dimensional network. Here, we report a three-dimensional network constructed via both projected covalent and O–H⋯O hydrogen bonding interactions: [(H2O)2Cu(pdc)] 1.
Results and discussion
Compound 1 was synthesized by reacting CuI with pyridine-3,5-dicarboxylic acid in the mol ratio of 1∶1 for 3 d at 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C under hydrothermal conditions. The pale-blue crystals of 1 obtained were suitable for single crystal X-ray diffraction analysis. Details of the crystal structure solutions and refinements are listed in Table 1.
°C under hydrothermal conditions. The pale-blue crystals of 1 obtained were suitable for single crystal X-ray diffraction analysis. Details of the crystal structure solutions and refinements are listed in Table 1.
| Parameter | |
|---|---|
| a Full-matrix, least squares refinement on F2. b Click b103981b.txt for full crystallographic data (CCDC 159941). | |
| Empirical formula | [(H2O)2CuNC5H3(CO2)2] |
| M | 264.68 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a/Å | 10.113(1) |
| b/Å | 11.859(1) |
| c/Å | 7.055(1) |
| β/° | 105.421(2) |
| V/Å3 | 815.65(10) |
| Z | 4 |
| T/K | 223(2) |
| λ/Å | 0.71073 |
| D c/g cm−3 | 2.155 |
| μ/mm−1 | 2.687 |
| Reflections collected | 2134 |
| Independent reflections | 762 (Rint⊕=⊕0.0295) |
| Goodness-of-fit on F2 | 1.097 |
| Final R indices [I⊕>⊕4σ(I)] | R 1⊕=⊕0.0206; wR2⊕=⊕0.0565 |
| R indices (all data) | R 1⊕=⊕0.0216; wR2⊕=⊕0.0572 |
The structure of 1 consists of trigonal bipyramidal copper centers (Fig. 1) coordinated by one pyridyl group and two carboxylate groups of pdc ligands at the equatorial positions, and two water molecules coordinate to the metal center at the axial positions (Fig. 2).
 | ||
| Fig. 1 View of the basic unit showing the atom numbering scheme. Thermal ellipsoids are 50% equiprobability envelopes, with hydrogen as spheres of arbitrary diameter. | ||
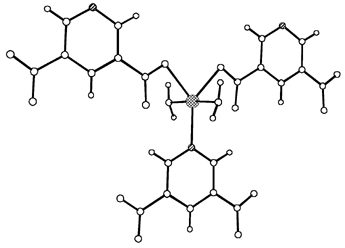 | ||
| Fig. 2 View of the complete trigonal bipyramidal coordination about the Cu atom. Click image or 2.htm to access a 3D representation. | ||
The network extends along the a- and b-axes (Fig. 3). This extended two-dimensional network consists of 20-membered large rings (Fig. 3) that are subsequently linked into a three-dimensional structure by inter-layer hydrogen bonds [O(3)–H⋯O(2) 2.694(2); O(3)–H⋯O(1) 2.745(2) Å] between the coordination water molecules and the carboxylate oxygen atoms in the adjacent layers (Fig. 4).
 | ||
| Fig. 3 View of the 2D network in the ab plane. | ||
 | ||
| Fig. 4 View down the b-axis of the hydrogen bonding between two adjacent layers. | ||
The Cu–O bonds at the axial positions are shorter than those at the equatorial positions, which should be expected, where copper atoms are in the +2 oxidation state. The oxidation reaction under hydrothermal conditions is rare but has been utilized recently to synthesize novel metal–organic polymers.16 Here in this reaction, the Cu(I) probably underwent disproportionation (auto-oxidation) reactions based on the potential diagram Cu2+ 0.153 V Cu+ 0.521 V Cu.17 The formation of 1 stabilizes the copper(II) under the reaction conditions.
It is noteworthy that the trigonal copper(II) centers coordinated by pdc observed in 1 are the first example synthesized of this type, although the trigonal coordination can be reasonably expected. The distance between the adjacent two-dimensional layers is ca. 3.4 Å. The aryl–aryl interactions may contribute a great deal to the stabilization of the three-dimensional structure.11b
Acknowledgements
The authors thank the Welch Foundation for financial support. This work made use of MRSEC/TCSUH Shared Experimental Facilities supported by the National Science Foundation and the Texas Center for Superconductivity at the University of Houston.References
- See, for example: R. Robson, B. F. Abrahams, S. R. Batten, R. W. Gable, B. F. Hoskins and J. Liu, Supramolecular Architecture, American Chemical Society, Washington, DC, 1992, ch. 19 CrossRef; M. O'Keeffe, M. Eddaoudi, H. Li, T. Reineke and O. M. Yaghi, J. Solid State Chem., 2000, 152, 3 CrossRef.
- See, for example: L. R. MacGillivray, S. Subramanian and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1994, 1325 RSC; S. Subramanian and M. J. Zaworotko, Coord. Chem. Rev., 1994, 137, 357 CrossRef CAS; O. M. Yaghi, H. Li, C. Davis, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef.
- See, for example: P. J. Stang, N. E. Persky and J. Manna, J. Am. Chem. Soc., 1997, 119, 4777 CrossRef CAS; S. W. Keller, Angew. Chem., Int. Ed. Engl., 1997, 36, 247 CrossRef.
- See, for example: S. W. Keller and S. Lopez, J. Am. Chem. Soc., 1999, 121, 6306 CrossRef CAS; J. A. Whiteford, C. V. Lu and P. J. Stang, J. Am. Chem. Soc., 1997, 119, 2524 CrossRef.
- D. Rabinowich and G. M. J. Schmidt, J. Chem. Soc. B, 1964, 144 Search PubMed.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1989, 111, 5692 CrossRef.
- See, for example: Vi T. Nguyen, R. Bishop, D. C. Craig and M. L. Scudder, CrystEngComm, 2000, 7; http://www.rsc.org/ej/ce/2000/a909836d/index.htm RSC; R. Bishop, Chem. Soc. Rev., 1996, 25, 311 RSC; D. Venkataraman, S. Lee, J. Zhang and J. S. Moore, Nature, 1994, 371, 591 CrossRef CAS.
- See, for example: P. Hubberstey, U. Suksangpanya and C. L. Wilson, CrystEngComm, 2000, 26; http://www.rsc.org/ej/ce/2000/b005851n/index.htm RSC; A. J. Blake, S. J. Hill, P. Hubberstey and W. Li, J. Chem. Soc., Dalton Trans., 1997, 913 RSC; P. V. Bernhardt, Inorg. Chem., 1999, 38, 3481 CrossRef CAS PubMed.
- G. R. Desiraju, Chem. Commun., 1997, 1475 RSC; J. M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, 1995 RSC; O. Ermer and A. Eling, J. Chem. Soc., Perkin Trans. 2, 1994, 925 RSC; O. Felix, M. W. Hosseini, A. De Cian and J. Fischer, Chem. Commun., 2000, 281 RSC.
- See, for example: B. Moulton and M. J. Zaworotko, Adv. Supramol. Chem., 2000, 7, 235 Search PubMed; M. J. Zaworotko, Angew. Chem., Int. Ed., 1998, 37, 1211 CrossRef CAS.
- See, for example: (a) A. D. Burrows, C.-W. Chan, M. M. Chowdhry, J. E. McGrady and D. M. P. Mingos, Chem. Soc. Rev., 1995, 24, 351 RSC; (b) A. S. Georgopoulou, D. M. P. Mingos, A. J. P. White, D. J. Williams, B. R. Horrocks, R. Benjamin and A. Houlton, J. Chem. Soc., Dalton Trans., 2000, 2969 RSC.
- (a) J. Y. Lu, C. Norman, K. A. Abboud and A. Ison, Inorg. Chem. Commun., 2001, accepted Search PubMed; (b) Y. M. Qian, A. Vogt, A. Vasudevan, S. M. Sebti and A. D. Hamilton, Bioorg. Med. Chem., 1998, 6, 293 CrossRef CAS PubMed.
- L. Ma, O. R. Evans, B. M. Foxman and W. Lin, Inorg. Chem., 1999, 38, 5837 CrossRef CAS; O. R. Evans and W. Lin, Inorg. Chem., 2000, 39, 2189 CrossRef PubMed.
- J. Y. Lu and A. M. Babb, Chem. Commun., 2001, 821 RSC; J. Y. Lu and A. M. Babb, Inorg. Chem., 2001, in press Search PubMed.
- Y. Sugiyama, K. Adachi, S. Kawata, H. Kumagai, K. Inoue, M. Katada and S. Kitagawa, CrystEngComm, 2000, 32; http://www.rsc.org/ej/ce/2000/B007939L/index.htm Search PubMed.
- J. Y. Lu and V. Schauss, J. Am. Chem. Soc., submitted Search PubMed.
- J. F. Rubinson and K. A. Rubinson, Contemporary Chemical Analysis, Prentice-Hall Inc., New Jersey, 1998 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |
