Enantiospecific single crystal-to-single crystal photocyclization of 2,5-diisopropyl-4′-carboxybenzophenone in the salt crystals with (S)- and (R)-phenylethylamine
Hideko
Koshima
*,
Daisuke
Matsushige
and
Masashi
Miyauchi
Department of Applied Chemistry, Faculty of Engineering, Ehime University, Matsuyama, 790-8577, Japan. E-mail: koshima@en3.ehime-u.ac.jp
Abstract
A salt crystal of 2,5-diisopropyl-4′-carboxybenzophenone and (S)-phenylethylamine underwent enantiospecific photocyclization via single crystal-to-single crystal transformation to give (R)-(+)-cyclobutenol in almost quantitative optical yield and 100% chemical yield.
Introduction
Asymmetric induction is efficiently achieved by solid state reaction of a chiral salt crystal formed from a carboxylic acid and an amine, one of which is a chiral compound acting as an ionic chiral handle.1–5 It is known that 2,4,6-triisopropyl-4′-carboxybenzophenone undergoes a Norrish type II photoreaction to give the cyclobutenol in the solid state and in the solution phase.6,7 Reaction in salt crystals formed from the 4′-carboxylic acid derivative with chiral amines, as an ionic chiral handle, produces the chiral cyclobutenol.8 Furthermore, single crystal-to-single crystal enantioselective photocyclization has already been achieved as the salt crystal with L-prolinol, but the problem remains in that the optical yield is low, only 30% ee.9 Herein we report the enantiospecific single crystal-to-single crystal reaction of 2,5-diisopropyl-4′-carboxybenzophenone 1, in the salt crystals 1·(S)-2 and 1·(R)-2, with (S)- and (R)-phenylethylamine 2 (Scheme 1). The reaction process was traced by X-ray crystallographic analysis. | ||
| Scheme 1 | ||
Results and discussion
Salt crystals of 1·(S)-2 (mp 159–164![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C) were prepared by crystallization from the methanol solution of both components. Irradiation of pulverized 1·(S)-2
(10 mg) with a 400 W high pressure mercury lamp through Pyrex glass under argon at 15
°C) were prepared by crystallization from the methanol solution of both components. Irradiation of pulverized 1·(S)-2
(10 mg) with a 400 W high pressure mercury lamp through Pyrex glass under argon at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C gave the chiral cyclobutenol (+)-3 as the sole product (Scheme 1). Optical yields were determined by HPLC using a chiral column;8Table 1 summarizes the results. The reaction proceeds smoothly, and almost constant optical yields of 91–92% ee are obtained at 22–100%
conversion of 1 with increasing irradiation time for 1–60 min. The opposite-handed crystals of 1·(R)-2 afforded (−)-3 also in almost constant optical yield of 90–96% ee at 23–100% conversion of 1. The [α]20D values of the products (+)-3 and (−)-3 were +205 (c 0.006, MeOH) and −204 (c 0.003, MeOH), respectively. Irradiation at −20
°C gave the chiral cyclobutenol (+)-3 as the sole product (Scheme 1). Optical yields were determined by HPLC using a chiral column;8Table 1 summarizes the results. The reaction proceeds smoothly, and almost constant optical yields of 91–92% ee are obtained at 22–100%
conversion of 1 with increasing irradiation time for 1–60 min. The opposite-handed crystals of 1·(R)-2 afforded (−)-3 also in almost constant optical yield of 90–96% ee at 23–100% conversion of 1. The [α]20D values of the products (+)-3 and (−)-3 were +205 (c 0.006, MeOH) and −204 (c 0.003, MeOH), respectively. Irradiation at −20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C increased the optical yields to 95 and 98% ee at conversions of 5 and 6% of 1
in 1·(S)-2 and 1·(R)-2, respectively, due to the decrease of thermal migration. These results suggest that the reaction might be single crystal-to-single crystal, and that it might proceed without decomposition of the initial crystal structure.
°C increased the optical yields to 95 and 98% ee at conversions of 5 and 6% of 1
in 1·(S)-2 and 1·(R)-2, respectively, due to the decrease of thermal migration. These results suggest that the reaction might be single crystal-to-single crystal, and that it might proceed without decomposition of the initial crystal structure.
| Crystal | Irradiation temperature/°C | Irradiation time/min | % Conversion of 1 | Product 3 (% ee) |
|---|---|---|---|---|
| 1·(S)-2 | 15 | 1 | 22 | (+) 91 |
| 15 | 10 | 51 | (+) 93 | |
| 15 | 60 | 100 | (+) 92 | |
| −20 | 120 | 5 | (+) 95 | |
| 1·(R)-2 | 15 | 1 | 23 | (−) 93 |
| 15 | 10 | 91 | (−) 96 | |
| 15 | 60 | 100 | (−) 90 | |
| −20 | 120 | 6 | (−) 98 |
After irradiation of single crystals of 1·(S)-2, the crystals were still transparent, confirming the single crystal-to-single crystal reaction. Finally, a piece of the single crystal of 1·(S)-2 (1.28⊕×⊕0.21⊕×⊕0.09 mm) was submitted for X-ray crystallographic analysis before and after successive irradiation times of 5, 15 and 45 min (Table 2). The data analyses were successful except for the 15 min irradiation. The reaction was completed upon 45 min irradiation; this indicates that (R)-3·(S)-2 is the product. The sizes of unit cells were not changed significantly after irradiation for 5 and 45 min: 0.332 Å (2.30%) and 0.635 Å (4.4%) decrease along the a axis; 0.043 Å (0.15%) and 0.222 Å (0.77%) decrease along the b axis; and 0.031 Å (0.50%) and 0.142 Å (2.3%) increase along the c axis, respectively. The crystals of 1·(S)-2 and (R)-3·(S)-2 are isomorphous to each other. The intermediate crystal 1a·(S)-2 is either a mixed crystal (solid solution) or a disordered crystal of 1·(S)-2 and (R)-3·(S)-2.
| Parameter | 1·(S)-2 | 1a·(S)-2 | (R)-3·(S)-2 |
|---|---|---|---|
| (Before irradiation) | (5 min irradiation) | (45 min irradiation) | |
| a Click b103517g.txt for full crystallographic data (CCDC 163081–163083). | |||
| Formula | C20H22O3·C8H11N | C20H22O3·C8H11N | C20H22O3·C8H11N |
| M | 431.57 | 431.57 | 431.57 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic |
| Space group | P212121 | P212121 | P212121 |
| a/Å | 14.411(1) | 14.079(2) | 13.776(1) |
| b/Å | 28.732(2) | 28.689(7) | 28.510(2) |
| c/Å | 6.1890(5) | 6.220(1) | 6.3312(3) |
| V/Å3 | 2562.6(4) | 2512.2(9) | 2486.6(3) |
| Z | 4 | 4 | 4 |
| μ(Cu-Kα)/mm−1 | 0.566 | 0.578 | 0.584 |
| D c/g cm−3 | 1.119 | 1.141 | 1.153 |
| Measured reflections | 2636 | 2425 | 2541 |
| Observed reflections [I⊕>⊕2.0σ(I)] | 1035 | 594 | 731 |
| Variables | 291 | 290 | 294 |
| R [I⊕>⊕2.0σ(I)] | 0.052 | 0.068 | 0.037 |
| R w | 0.153 | 0.109 | 0.109 |
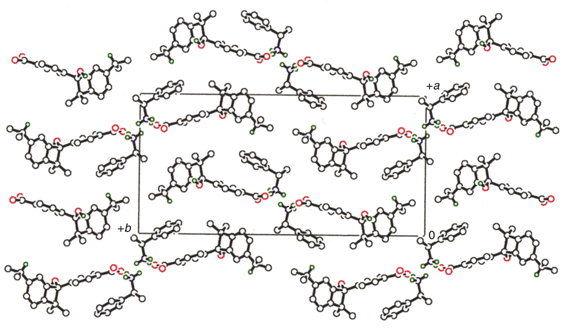
Fig. 1 shows the molecular arrangement in the reactant 1·(S)-2. Two molecules of each component are connected through an N+H3⋯−O quarternary ammonium salt bridge between the carboxy group of 1 and the amino group of (S)-2 to form a two-fold screw axis along the c axis. The H1Nb⋯O2, H1Nc⋯O2 and H1Na⋯O3 distances are 1.87, 1.89 and 1.78 Å (see atom numbering in Fig. 3). The molecular packing in the product (R)-3·(S)-2 (Fig. 2) is very similar to that in the reactant 1·(S)-2 (Fig. 1), giving a visual understanding for the single crystal-to-single crystal reaction. The initial salt bridge is kept in the lattice of the product.
 | ||
| Fig. 1 Molecular arrangement in the reactant 1·(S)-2 before irradiation. All hydrogen atoms except isopropyl methine hydrogens, carboxy and amine hydrogens are omitted for clarity. | ||
 | ||
| Fig. 2 Molecular arrangement in the product (R)-3·(S)-2 after irradiation for 45 min. All hydrogen atoms except isopropyl methine hydrogens, carboxy and amine hydrogens are omitted for clarity. | ||
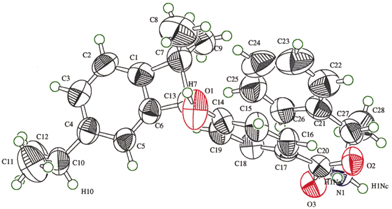
Ortep drawings of salt bonding pairs in 1·(S)-2 and (R)-3·(S)-2 are shown in Figs. 3 and 4, respectively. Irradiation of the reactant 1·(S)-2 excites the carbonyl group C13![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O1 of the benzophenone unit and abstracts enantiospecifically the methine hydrogen H7 of the o-isopropyl group, despite the O1⋯H7 distance (3.15 Å) which seems to be slightly longer than 2.2–2.7 Å as proposed by Scheffer.10 In contrast, another hydrogen abstraction from the m-isopropyl
group does not occur due to the long O1⋯H10 distance (5.19 Å).
O1 of the benzophenone unit and abstracts enantiospecifically the methine hydrogen H7 of the o-isopropyl group, despite the O1⋯H7 distance (3.15 Å) which seems to be slightly longer than 2.2–2.7 Å as proposed by Scheffer.10 In contrast, another hydrogen abstraction from the m-isopropyl
group does not occur due to the long O1⋯H10 distance (5.19 Å).
 | ||
| Fig. 3 Ortep drawing of the salt pair in the reactant 1·(S)-2 before irradiation. Click image or 3.htm to access a 3D representation. | ||
 | ||
| Fig. 4 Ortep drawing of the salt pair in the product (R)-3·(S)-2 after irradiation for 45 min. Click image or 4.htm to access a 3D representation. | ||
Upon only 5 min of irradiation, the H7 hydrogen atom was not found, the C13⋯C7 distance (2.97 Å) between the carbonyl carbon and the methine carbon in 1·(S)-2 was shortened to 2.56 Å, and the bond angles of C13–C6–C1 [120.6(5)°] and C6–C1–C7 [122.0(5)°] decreased to 112(1) and 112(1)° in 1a·(S)-2. Subsequently, the ˙C13 and ˙C7 biradicals were enantiospecifically coupled to produce an enantiomorphic cyclobutenol 3 after 45 min irradiation (Fig. 4). The cyclobutene ring C13–C7–C1![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) C6 is highly strained because the
C13–C7 single bond length [1.64(1) Å] is significantly longer than the usual C–C bond length (ca. 1.5 Å). The absolute configuration of cyclobutenol 3 was confirmed to be (R) by comparison with the known absolute configuration of (S)-2 from X-ray crystallographic analysis. This means that 1·(S)-2 gives the enantiomorphic (R)-(+)-3 as the sole product; conversely, 1·(R)-2 affords (S)-(
−)-3.
C6 is highly strained because the
C13–C7 single bond length [1.64(1) Å] is significantly longer than the usual C–C bond length (ca. 1.5 Å). The absolute configuration of cyclobutenol 3 was confirmed to be (R) by comparison with the known absolute configuration of (S)-2 from X-ray crystallographic analysis. This means that 1·(S)-2 gives the enantiomorphic (R)-(+)-3 as the sole product; conversely, 1·(R)-2 affords (S)-(
−)-3.
Such a photocyclization, however, did not significantly change the molecular conformation and arrangement as a whole within the lattice. The dihedral angles between the diisopropylbenzene plane and the carboxybenzene plane are 89.8(3) and 77.8(3)° in the reactant and product, respectively. The dihedral angles between the carboxybenzene plane of 1 or 3 and the phenyl plane of (S)-2 are 47.9(3) and 25.9(4)° in 1·(S)-2 and (R)-3·(S)-2, respectively. It is thought that the strong salt bonding contributes to fixing of the molecules during the reaction to lead to reaction without decomposition of the initial crystal structure.
In the case of the single crystal-to-single crystal reaction in the salt crystal of 2,4,6-triisopropyl-4′-carboxybenzophenone with L-prolinol, the optical yield was low (30% ee) due to the pseudo mirror image-related arrangement of the benzophenone moiety in the asymmetric unit.8,9
In conclusion, we achieved high optical yields using the salt crystals of 2,5-diisopropyl-4′-carboxybenzophenone with (S)- and (R)-phenylethylamine, and the enantiospecific reaction paths via the hydrogen abstraction and the radical coupling were elucidated based on the single crystal-to-single crystal photoreaction by X-ray crystallographic analysis.
Crystallographic studies
X-Ray data were collected on a Rigaku RAXIS RAPID imaging plate diffractometer with Cu-Kα radiation to a maximum 2θ of 136.5°. A total of 30⊕×⊕5.00° oscillation images was collected, each being exposed for 30.0 min. The reflection data were corrected for Lorentz-polarization effects and secondary extinction. The structures were solved by direct methods and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically and hydrogen atoms were not refined. The final cycle of full-matrix, least squares refinement was based on all reflections (2θ⊕<⊕136.5°) and variable parameters, and converged with unweighted and weighted agreement factors of: R⊕=⊕Σ||Fo|−|Fc||/Σ|Fo| for I⊕>⊕2.0σ(I) and Rw⊕=⊕{Σw(Fo2−Fc2)2/Σw(Fo2)2}1/2. All calculations were performed using the teXsan crystallographic software package.11 The absolute structures for 1·(S)-2, 1a·(S)-2 and (R)-3·(S)-2 were determined by reference to the known configuration of the chiral amine molecule (S)-2.Acknowledgements
We thank Dr M. Shiro of the Rigaku Corporation for help with the X-ray data analysis. This work was supported by a Grant-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.References
- D. Gudmundsdottir and J. R. Scheffer, Tetrahedron Lett., 1990, 31, 6807 CrossRef.
- D. Gudmundsdottir and J. R. Scheffer, Photochem. Photobiol., 1991, 54, 535 CrossRef.
- J. R. Scheffer, R. Trotter and J. Yang, Tetrahedron Lett., 1992, 33, 5481 CrossRef.
- D. Gudmundsdottir, J. R. Scheffer and J. Trotter, Tetrahedron Lett., 1994, 35, 1397 CrossRef.
- J. N. Gamlin, R. Jones, M. Leibovitch, B. Patrick, J. R. Scheffer and J. Trotter, Acc. Chem. Res., 1996, 29, 203 CrossRef CAS.
- Y. Ito, H. Nishimura, Y. Umehara, Y. Yamada, M. Tone and T. Matsuura, J. Am. Chem. Soc., 1983, 105, 1590 CrossRef CAS.
- Y. Ito and T. Matsuura, Tetrahedron Lett., 1988, 29, 3087 CrossRef CAS.
- H. Koshima, A. Maeda, T. Matsuura, K. Hirotsu, K. Okada, H. Mizutani, Y. Ito, T. Y. Fu, J. R. Scheffer and J. Trotter, Tetrahedron: Asymmetry, 1994, 5, 1415 CrossRef CAS.
- K. Hirotsu, K. Okada, H. Mizutani, H. Koshima and T. Matsuura, Mol. Cryst. Liq. Cryst., 1996, 277, 99 CrossRef.
- J. F. Scheffer, in Organic Solid State Chemistry, ed. G. R. Desiraju, Elsevier, New York, 1987, pp. 1–45 Search PubMed.
- TeXsan, Single Crystal Structure Analysis Software, Version 1.11 , Molecular Structure Corporation, The Woodlands, TX, 1995 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |
