Polymeric versus monomeric and tetrahedral versus octahedral coordination in zinc(II) pyridine complexes
Chunhua
Hu
and
Ulli
Englert
*
Institut für Anorganische Chemie, RWTH Aachen, Prof.-Pirlet-Str. 1, 52074, Aachen, Germany. E-mail: ullrich.englert@ac.rwth-aachen.de
Abstract
The first one-dimensional chain polymers of the type [Zn(μ-Cl)2py]∞ (py⊕=⊕3,5-dichloropyridine and 3,5-dibromopyridine) were synthesized by reaction of the 3,5-dihalopyridines with ZnCl2. In the resulting linear coordination polymer octahedral zinc(II) centres are linked in an edge-sharing fashion by halogen bridges in their pseudo-equatorial plane. In contrast to these findings the analogous reaction between ZnBr2 and the 3,5-dihalopyridines results in the formation of mononuclear tetrahedral complexes. Both of the zinc dihalides react with 3,4,5-trichloropyridine with formation of isotypic molecular crystals which show relatively short halogen⋯halogen contacts; their projections along the [001] direction exhibit a remarkable similarity to those of the chain polymers. From ZnX2 (X⊕=⊕Cl, Br) and 4,4′-bipyridyl (bpy) a zigzag chain coordination polymer of composition [ZnX2(μ-bpy)]∞ was obtained.
Introduction
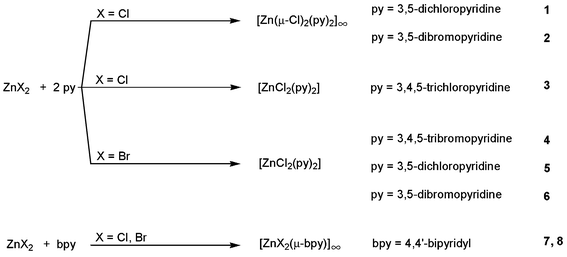
Over the past few years considerable efforts have been devoted to the synthesis and structural characterization of coordination polymers. Studies involving zinc(II) resulted in reports on chain polymers,1–5 two-dimensional layer structures6,7 and three-dimensional frameworks.8A general search of the Cambridge Structural Database (CSD)9 for zinc complexes of any nuclearity confirms that coordination numbers of 4, 5 and 6 are commonly encountered.10 Here, we report eight new crystal structures involving the coordination of pyridine derivatives to zinc(II) halides (Scheme 1).
 | ||
| Scheme 1 | ||
Our selection is intended to demonstrate the interplay between coordination geometry, steric requirements of the ligands, and intermolecular interactions. Issues of packing and space filling will be addressed.
Experimental
Chemicals and reagents
All chemicals were used as purchased without purification: ZnCl2 (98%, Merck), ZnBr2 (98%, Fluka), 3,5-dichloropyridine (3,5-Cl2py) (98%, Aldrich), 3,5-dibromopyridine (3,5-Br2py) (99%, Aldrich), 3,4,5-trichloropyridine (3,4,5-Cl3py) (98%, Aldrich), 4,4′-bipyridyl (4,4′-bpy) (99%, Merck).Synthesis of [Zn(μ-Cl)2(3,5-Cl2py)2]∞ (1)
Reaction of ZnCl2 (4 mmol, 0.545 g) and 3,5-Cl2py (8 mmol, 1.184 g) in 50 ml EtOH gave a colourless solution and a white precipitate. Colourless crystals of 1 were obtained by evaporation of the filtrate in air. A second crop of crystals can be obtained by evaporating a solution of the original precipitate in acetone. Overall yield quantitative. 1H NMR (200 MHz, d6-acetone): δ 8.09 (m, 1H, H4), 8.58 (d, 2H, H2,6).Synthesis of [Zn(μ-Cl)2(3,5-Br2py)2]∞ (2)
Similar to the synthesis of 1, the reaction of ZnCl2 (4 mmol, 0.545 g) and 3,5-Br2py (8 mmol, 1.896 g) in 100 ml EtOH gave colourless crystals of 2 in quantitative yield. 1H NMR (200 MHz, d6-acetone): δ 8.37 (m, 1H, H4), 8.71 (d, 2H, H2,6).Synthesis of [ZnCl2(3,4,5-Cl3py)2] (3)
Reaction of ZnCl2 (2 mmol, 0.273 g) and 3,4,5-Cl3py (4 mmol, 0.730 g) in 50 ml EtOH gave colourless crystals of 3 in quantitative yield. 1H NMR (200 MHz, d6-acetone): δ 8.67 (s, 2H, H2,6).Synthesis of [ZnBr2(3,4,5-Cl3py)2] (4)
Reaction of ZnBr2 (2 mmol, 0.450 g) and 3,4,5-Cl3py (4 mmol, 0.730 g) in 50 ml EtOH occurred without obvious phenomenon. Colourless crystals of 4 were grown by slow evaporation of the solution. Yield quantitative. 1H NMR (200 MHz, d6-acetone): δ 8.68 (s, 2H, H2,6).Synthesis of [ZnBr2(3,5-Cl2py)2] (5)
By analogy to the synthesis of 4, reaction of ZnBr2 (4 mmol, 0.901 g) and 3,5-Cl2Py (8 mmol, 1.184 g) in 100 ml EtOH occurred without obvious phenomenon. Colourless crystals of 5 were crystallized by slow evaporation of the solution. Yield quantitative. 1H NMR (200 MHz, d6-acetone): δ 8.18 (m, 1H, H4), 8.63 (d, 2H, H2,6).Synthesis of [ZnBr2(3,5-Br2py)2] (6)
Similar to the syntheses of 4 and 5, reaction of ZnBr2 (4 mmol, 0.901 g) and 3,5-Br2py (8 mmol, 1.896 g) in 100 ml EtOH gave colourless crystals of 6 in quantitative yield. 1H NMR (200 MHz, d6-acetone): δ 8.40 (m, 1H, H4), 8.73 (d, 2H, H2,6).Synthesis of [ZnCl2(μ-4,4′-bpy)]∞ (7)
Reaction of ZnCl2 (6 mmol, 0.818 g) and 4,4′-bpy (6 mmol, 0.937 g) in 50 ml EtOH gave a white solid. Crystals of 7 were obtained in quantitative yield by evaporating a solution of the solid in DMSO. Alternatively, crystals of 7 may be grown by sublimation of the powder at ca. 310 °C in vacuo. 1H NMR (200 MHz, d6-DMSO): δ 7.83 (dd, 4H, H3,5,3′,5′), 8.72 (dd, 4H, H2,6,2′,6′).Synthesis of [ZnBr2(μ-4,4′-bpy)]∞ (8)
Similar to the synthesis of 7, reaction of ZnBr2 (6 mmol, 1.351 g) and 4,4′-bpy (6 mmol, 0.937 g) in 50 ml EtOH gave a white solid in quantitative yield. Crystals of 8 were obtained from DMSO–acetone solution. 1H NMR (200 MHz, d6-DMSO): δ 7.83 (dd, 4H, H3,5,3′,5′), 8.72 (dd, 4H, H2,6,2′,6′).Crystallographic studies
Compounds 1–8 are air-stable and colourless. Crystals were mounted on glass fibers. Geometry and intensity data were obtained using a Bruker SMART Apex CCD area detector diffractometer. Preliminary lattice parameters and orientation matrices were obtained from three sets of frames. They were re-refined during the integration process of the intensity data. All data were collected using graphite-monochromated Mo-Kα radiation (λ⊕=⊕0.71073 Å) at 293(2) K with the ω scan method.11 Data were processed using the SAINT+ program.12 Empirical absorption corrections were applied to all data sets by using the SADABS program for area detector data.13 All structures were solved by direct methods and refined using SHELXTL.14 Non-hydrogen atoms were refined with anisotropic displacement parameters, and hydrogen atoms were placed in idealized positions (C–H⊕=⊕0.98 Å) and included as riding with Uiso(H)⊕=⊕1.3 Ueq(non-H). Crystal data, data collection parameters and refinement results for 1–8 are listed in Tables 1 and 2.| Parameter | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| a Click b103378f(a).txt for full crystallographic data (CCDC 162045–162048). | ||||
| Empirical formula | C10H6Cl6N2Zn | C10H6Cl2Br4N2Zn | C10H4Cl8N2Zn | C10H4Br2Cl6N2Zn |
| Crystal dimensions/mm | 0.42⊕×⊕0.12⊕×⊕0.10 | 0.50⊕×⊕0.22⊕×⊕0.19 | 0.58⊕×⊕0.22⊕×⊕0.16 | 0.50⊕×⊕0.12⊕×⊕0.06 |
| M | 432.24 | 610.08 | 501.12 | 590.04 |
| Crystal shape | Rod | Rod | Rod | Needle |
| Crystal system | Tetragonal | Tetragonal | Tetragonal | Tetragonal |
| Space group (no.) |
P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) b2 (117) b2 (117) |
P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) b2 (117) b2 (117) |
P42nm (102) | P42nm (102) |
| a/Å | 13.846(3) | 13.888(3) | 12.6282(11) | 12.527(4) |
| c/Å | 3.6542(10) | 3.7300(12) | 5.2467(7) | 5.549(2) |
| V/Å3 | 700.5(3) | 719.5(3) | 836.70(15) | 870.8(5) |
| Z | 2 | 2 | 2 | 2 |
| D c/g cm−3 | 2.049 | 2.816 | 1.989 | 2.250 |
| μ/mm−1 | 28.80 | 131.69 | 27.35 | 69.10 |
| F(000) | 424 | 568 | 488 | 560 |
| Scan range (θ)/° | 2–28 | 3–28 | 3–28 | 3–28 |
| Total reflections | 4037 | 9405 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 972 972 |
6991 |
| Unique reflections | 820 | 890 | 1098 | 1127 |
| Variables refined | 52 | 52 | 60 | 60 |
| Flack parameter | 0.02(2) | 0.015(17) | 0.028(14) | 0.07(3) |
| R 1 [I⊕>⊕2σ(I)] | 0.0268 | 0.0207 | 0.0223 | 0.0631 |
| wR 2 (all reflections) | 0.0593 | 0.0460 | 0.0614 | 0.1480 |
| Residual electron density/e Å−3 | 0.396 | 0.675 | 0.465 | −1.528 (close to Br) |
| Parameter | 5 | 6 | 7 | 8 |
|---|---|---|---|---|
| a Click b103378f(b).txt for full crystallographic data (CCDC 162049–162052). | ||||
| Empirical formula | C10H6Br2Cl4N2Zn | C10H6Br6N2Zn | C10H8Cl2N2Zn | C10H8Br2N2Zn |
| Crystal dimensions/mm | 0.42⊕×⊕0.27⊕×⊕0.13 | 0.45⊕×⊕0.30⊕×⊕0.20 | 0.35⊕×⊕0.08⊕×⊕0.06 | 0.67⊕×⊕0.12⊕×⊕0.08 |
| M | 521.16 | 699.00 | 292.45 | 381.37 |
| Crystal shape | Block | Prism | Needle | Needle |
| Crystal system | Monoclinic | Triclinic | Monoclinic | Monoclinic |
| Space group (no.) | P21/c (14) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2)
(2) |
C2/c (15) | C2/c (15) |
| a/Å | 12.547(2) | 8.0886(9) | 15.830(2) | 15.989(5) |
| b/Å | 11.0055(19) | 9.6282(11) | 5.1132(8) | 5.4211(7) |
| c/Å | 13.142(2) | 11.1754(12) | 14.5963(18) | 15.0100(19) |
| α/° | — | 88.184(3) | — | — |
| β/° | 117.262(3) | 82.233(2) | 110.265(2) | 113.440(3) |
| γ/° | — | 82.338(2) | — | — |
| V/Å3 | 1613.1(5) | 854.56(16) | 1108.3(2) | 1193.6(3) |
| Z | 4 | 2 | 4 | 4 |
| D c/g cm−3 | 2.146 | 2.717 | 1.753 | 2.122 |
| μ/mm−1 | 71.24 | 154.51 | 26.62 | 87.19 |
| F(000) | 992 | 640 | 584 | 728 |
| Scan range (θ)/° | 2–28 | 2–28 | 2–28 | 2–28 |
| Total reflections | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 739 739 |
6589 | 7088 | 4195 |
| Unique reflections | 4005 | 4171 | 1374 | 1476 |
| Variables refined | 173 | 173 | 69 | 69 |
| Extinction coefficient/Å−3 | 0.0215(5) | 0.0175(4) | — | — |
| R 1 [I⊕>⊕2σ(I)] | 0.0309 | 0.0341 | 0.0529 | 0.0470 |
| wR 2 (all reflections) | 0.0645 | 0.0694 | 0.1292 | 0.1271 |
| Residual electron density/e Å−3 | 0.619 | 0.681 | 0.640 | −0.933 |
Results and discussion
When ZnCl2 or ZnBr2 are reacted with pyridine derivatives the formation of mononuclear tetrahedral complexes can be expected. The original interest of our work in this field was the comparison of packing modes, intermolecular interactions and space filling properties for these presumably simple complexes of zinc(II) with pyridine derivatives. In addition to published examples15–26 we indeed obtained and fully characterized products of this type from reactions with unsubstituted pyridine or its derivatives 2-halopyridine, 3-halopyridine, 4-halopyridine or 3,5-dimethylpyridine. As they do not exhibit exceptionally short intermolecular interactions and do not show obvious structural relationships with the compounds discussed in the following section, their crystal and molecular structures will be reported in a forthcoming contribution. To our surprise, reaction between ZnCl2 and 3,5-dichloropyridine or 3,5-dibromopyridine resulted in the formation of the coordination polymers 1 and 2. A section of polymer 1 is represented in Fig. 1.27![Displacement ellipsoid plot27 of a section of coordination polymer [Zn(μ-Cl)2(3,5-Cl2py)2]∞, 1. Ellipsoids are scaled to 50% probability, H atoms are represented with arbitrary radius. Click image or here to access a 3D representation.](/image/article/2001/CE/b103378f/b103378f-f1.gif) | ||
| Fig. 1 Displacement ellipsoid plot27 of a section of coordination polymer [Zn(μ-Cl)2(3,5-Cl2py)2]∞, 1. Ellipsoids are scaled to 50% probability, H atoms are represented with arbitrary radius. Click image or 1.htm to access a 3D representation. | ||
Although this type of polymeric chain is commonly encountered for complexes of the higher homologue cadmium(II),28–31 to the best of our knowledge 1 and 2 represent the first examples of a one-dimensional coordination polymer based on halogen-bridged edge-sharing zinc(II) octahedra. The only precedents for an arrangement similar to that described above are represented by the two-dimensional layer structures [ZnCl2(py)] (py⊕=⊕pyrazine, pyrimidine)7 which are isotypic to the corresponding Cd derivatives.29,30 In the structure of 1 chains of chloro-bridged Zn(II) octahedra extend along the [001] direction with the short, tetragonal c-axis of 3.6542(10) Å representing the Zn⋯Zn vector. The apical positions in the pseudo-octahedron around each metal center are occupied by the nitrogen atoms of the donor ligands. The ring planes between the pyridine ligands bonded to the same Zn cation are tilted by an angle of 36°. This ensures a short contact of 2.69 Å between the ortho hydrogen atom of the pyridine ring and its closest metal-bridging Cl neighbor. A similar geometry has been observed in the structure of [CdCl2(py)] (py⊕=⊕pyrazine) by Bailey and Pennington29 who attributed this deviation from a hypothetical mmm symmetry to non-classical C–H⋯Cl hydrogen bonds.32 An important feature of our coordination polymer are the contacts between neighboring pyridine rings along the same chain which amount to a lattice translation along [001], i.e. a distance of 3.6542(10) Å. They involve stacking of the aromatic rings as well as close interchlorine contacts. For the spherical van der Waals (vdW) radius of chlorine, values of 1.7633 and 1.8 Å34 have been proposed; if the concept of polar flattening is accepted, a radius of 1.78 Å applies.35 In agreement with these numbers the intramolecular Cl⋯Cl distances in 1 may be interpreted as favourable vdW interactions.
Fig. 2(a) 36 illustrates the packing of the polymeric chains in the projection on the ab plane.
![Projection36 of the unit cells of (a) the polymer [Zn(μ-Cl)2(3,5-Cl2py)2]∞, 1, and (b)
[ZnCl2(3,4,5-Cl3py)2], 3, along [001].](/image/article/2001/CE/b103378f/b103378f-f2.gif) | ||
| Fig. 2 Projection36 of the unit cells of (a) the polymer [Zn(μ-Cl)2(3,5-Cl2py)2]∞, 1, and (b) [ZnCl2(3,4,5-Cl3py)2], 3, along [001]. | ||
Two types of relatively short intermolecular interactions are worth mentioning: interactions between chlorine substituents, bonded to adjacent polymer strands and symmetry-related by action of the fourfold inversion axis, occur at a distance of 3.77 Å; and C–H⋯Cl contacts of 2.82 Å exist between the para hydrogen atom of the pyridine ring and the closest metal-bridging Cl ligands of a neighboring chain. The latter interactions, translated along [001], result in a zipper-like, interchain arrangement of the C–H groups of one [marked as ‘A’ in Fig. 2(a)] and the chloro ligands (marked ‘B’) of an adjacent polymer strand.
In the isotypic compound 2 the translation period along the chain is slightly longer and amounts to 3.7300(12) Å, as might be expected when the carbon-bonded chlorine substituents are replaced by the higher homologue bromine (vdW radius 1.85,33 1.9,34 or 1.84 Å35). The tilt angle of 42° between the two pyridine rings bonded to each metal and the interchain C–H⋯Cl contacts of 2.87 Å are similar to the values found for 1.
Space filling in the coordination polymers is highly efficient. When Gavezzotti's sampling method37 is applied, packing coefficients of 0.767 for 1 and 0.793 for 2 are calculated. The favourable packing may also be visualized with the help of a model of a polymer chain of 2 in which the atoms are represented by their vdW spheres (Fig. 3).
![Space-filling model36 of the polymer [Zn(μ-Cl)2(3,5-Br2py)2]∞, 2. Color code: C, black; H, magenta; N, blue; Br, yellow; Cl, green; Zn, grey.](/image/article/2001/CE/b103378f/b103378f-f3.gif) | ||
| Fig. 3 Space-filling model36 of the polymer [Zn(μ-Cl)2(3,5-Br2py)2]∞, 2. Color code: C, black; H, magenta; N, blue; Br, yellow; Cl, green; Zn, grey. | ||
Zinc dichloride and zinc bromide react with 3,4,5-trichloropyridine resulting in the formation of mononuclear tetrahedral complexes. The resulting isotypic products 3 and 4 crystallize in space group P42nm with the metal in special positions 2a, corresponding to an arrangement of objects with 2mm
(C2v) site symmetry which are packed according to the symmetry operations in subgroup P21. Projections of 3 and 4 along the principal symmetry direction show remarkable similarity to those of the polymers 1 and 2
(Fig. 2). All four complexes crystallize in tetragonal space groups. The special projections
along [001] for P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) b2 (coordination polymers 1 and 2) and P42nm
(mononuclear complexes 3 and 4) belong to the plane group p4gm, albeit with different origin,38 and hence comparable intermolecular arrangements with a component in the ab plane occur. The relatively short interhalogen contacts around the fourfold rotation points are similar in both structures and are in agreement with expectations for vdW crystals. However, the H⋯Cl interactions in 1 and 2 have their equivalents in Cl⋯Cl [3.26 Å, 3, emphasized as hollow green ‘bonds’
in Fig. 2(b)] or Cl⋯Br (3.40 Å, 4) interactions which are considerably shorter than the sum of the vdW radii. Interhalogen contacts of this type are attractive according to Desiraju and Parthasarathy.39
b2 (coordination polymers 1 and 2) and P42nm
(mononuclear complexes 3 and 4) belong to the plane group p4gm, albeit with different origin,38 and hence comparable intermolecular arrangements with a component in the ab plane occur. The relatively short interhalogen contacts around the fourfold rotation points are similar in both structures and are in agreement with expectations for vdW crystals. However, the H⋯Cl interactions in 1 and 2 have their equivalents in Cl⋯Cl [3.26 Å, 3, emphasized as hollow green ‘bonds’
in Fig. 2(b)] or Cl⋯Br (3.40 Å, 4) interactions which are considerably shorter than the sum of the vdW radii. Interhalogen contacts of this type are attractive according to Desiraju and Parthasarathy.39
In contrast to the above-mentioned formation of coordination polymers 1 and 2 from ZnCl2 and the 3,5-dihalopyridines, the analogous reactions between these ligands and ZnBr2 result in formation of the mononuclear tetrahedral complexes 5 and 6. A search in the CSD revealed that no structures involving six coordinated zinc(II) centers with more than two bromo ligands coordinated to the same metal cation have been reported: probably, structures equivalent to 1 and 2, i.e. hypothetical bromo-bridged one-dimensional coordination polymers, cannot form for steric reasons. The mononuclear reaction products 5 and 6 are represented in Fig. 4.
![Displacement ellipsoid plot27 of (left)
[ZnBr2(3,5-Cl2py)2], 5 and (right)
[ZnBr2(3,5-Br2py)2], 6. Ellipsoids are scaled to 50% probability, H atoms are represented with arbitrary radius.](/image/article/2001/CE/b103378f/b103378f-f4.gif) | ||
| Fig. 4 Displacement ellipsoid plot27 of (left) [ZnBr2(3,5-Cl2py)2], 5 and (right) [ZnBr2(3,5-Br2py)2], 6. Ellipsoids are scaled to 50% probability, H atoms are represented with arbitrary radius. | ||
Non-classical C–H⋯Br interactions represent the shortest intermolecular contacts in both structures with the H atom in a position para to one of the pyridine ligands approaching a bromo ligand of a neighboring molecule. The intermolecular distances associated with these interactions are 2.94 Å in 5 and 2.74 Å in 6. No short halogen⋯halogen contacts occur in these structures. Packing coefficients amount to 0.633 for 5 and 0.708 for the bis(3,5-dibromopyridine) complex 6 and cannot compete with the high numbers found for the polymers 1 and 2.
In structures 1–6 octahedral coordination of zinc(II) is associated with the formation of polymeric species whereas tetrahedral geometry is observed for mononuclear complexes. Reaction of the zinc dihalides ZnCl2 and ZnBr2 with 4,4′-bipyridyl results in quantitative formation of the coordination polymers 7 and 8, respectively. In these isotypic compounds bipyridyl ligands bridge ZnX2 moieties with an essentially tetrahedral arrangement at the metal. Fig. 5 shows a section of the resulting zigzag chain in [ZnCl2(μ-bpy)]∞, 7.
![Displacement ellipsoid plot27 of a section of the coordination polymer [ZnCl2(μ-bpy)]∞, 7. Ellipsoids are scaled to 50% probability, H atoms are represented with arbitrary radius. Click image or here to access a 3D representation.](/image/article/2001/CE/b103378f/b103378f-f5.gif) | ||
| Fig. 5 Displacement ellipsoid plot27 of a section of the coordination polymer [ZnCl2(μ-bpy)]∞, 7. Ellipsoids are scaled to 50% probability, H atoms are represented with arbitrary radius. Click image or 5.htm to access a 3D representation. | ||
The coordination polymer extends along the [101] direction, with the Zn atoms on twofold crystallographic axes and the midpoints of the C3–C3′ bonds on centers of inversion. Slightly elongated displacement ellipsoids for the carbon atoms C1, C2, C4 and C5 indicate either librational movement or static disorder with respect to the dihedral angle between the two aromatic rings of the bipyridine ligand. Structures of similar connectivity have been described for thiolato4 and dithiophosphato1 groups as monoanionic ligands, and in the case of [Zn(NCS)2(μ-bpy)]∞3 an isotypic coordination polymer has been obtained. We note that crystals of 7 may be grown via both sublimation and isothermic evaporation of a saturated solution in DMSO.
In this article we have communicated a series of crystal structures for pyridine complexes of zinc(II) halides comprising two very different types of coordination polymer: for 7 and 8 with the rigid bridging 4,4′-bipyridyl moiety the formation of chain polymers can be predicted, whereas the existence of coordination polymers based on octahedral zinc(II), 1 and 2, seems to be limited to the special combination of chlorine as bridging atoms and 3,5-dihalogen pyridine ligands in the apical positions. Replacement of the (μ)-Cl ligands by bromine bridges, insertion of an additional halogen substituent in the 4-position, or removal of one or both of the 3- and 5-halogen atoms of the pyridine ring as well as exchange of these halogen moieties for methyl groups will result in mononuclear species. We note that reaction of the zinc dihalides with 2,6-dichloropyrazine, a ligand which cannot bridge due to the steric congestion around the nitrogen atom at the 1-position and which might be expected to have approximately the same steric requirements as 3,5-dichloropyrdine, also gives mononuclear complexes.40 When compared to the analogous cadmium complexes, a more subtle balance between steric requirements of the ligands and intermolecular interactions has to be met than is the case for formation of octahedral zinc(II) coordination polymers.
Acknowledgements
Support from Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie is gratefully acknowledged. The authors wish to thank Mrs X. Liu for friendly help.References
- D.-L. Zhu, Y.-P. Yu, G.-C. Guo, H.-H. Zhung, J.-S. Hung, Q. Liu, Z. Xu and X.-Z. You, Acta Crystallogr. Sect. C, 1996, 52, 1963 CrossRef.
- M. Barquin, J. Cancela, M. J. Gonzalez Garmendia, J. Quintanilla and U. Amador, Polyhedron, 1998, 17, 2372 CrossRef.
- M. Kondo, M. Shimamura, S. Noro, T. Yoshitomi, S. Minakoshi and S. Kitagawa, Chem. Lett., 1999, 285 CrossRef CAS.
- J. T. Sampanthar and J. J. Vittal, J. Chem. Soc., Dalton Trans., 1999, 1993 RSC.
- O. R. Evans and W. Lin, Cryst. Growth Des., 2001, 1, 9 Search PubMed.
- J. Y. Lu, M. A. Lawandy, J. Li, T. Yuen and C. L. Lin, Inorg. Chem., 1999, 38, 2695 CrossRef CAS.
- J. Pickardt and B. Staub, Z. Naturforsch., B: Chem. Sci., 1996, 51, 947 CAS.
- S. Subramanian and M. J. Zaworotko, Angew. Chem., Int. Ed. Engl., 1995, 34, 2127 CrossRef CAS.
- F. H. Allen and O. Kennard, Chem. Des. Automat. News, 1993, 8, 31 Search PubMed.
- In March 2001, a search for zinc complexes (error-free coordinates available, R⊕<⊕0.1) resulted in 1371 complexes of coordination number 4, 551 complexes of coordination number 5, and 548 complexes of coordination number 6 out of a total of 224
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 entries.
400 entries. - SMART (version 5.618), Program for Bruker CCD X-Ray Diffractometer Control , Bruker AXS Inc., Madison, WI, 1999 Search PubMed.
- SAINT+ (version 6.02), Program for Reduction of Data Collected on a Bruker CCD Area Detector Diffractometer , Bruker AXS Inc., Madison, WI, 1999 Search PubMed.
- G. M. Sheldrick, SADABS, Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, SHELXTL, version 5.1, Bruker Analytical X-ray Systems, Inc., Madison, WI, 1998 Search PubMed.
- S. Zanetti, Gazz. Chim. Ital., 1960, 90, 328 Search PubMed.
- H. Lynton and M. C. Sears, Can. J. Chem., 1971, 49, 3418 CrossRef CAS.
- L. Fanfani, A. Nunzi and P. F. Zanazzi, Acta Crystallogr., Sect. B, 1972, 28, 323 CrossRef CAS.
- M. Laing, Acta Crystallogr., Sect. A, 1975, 31, S147 Search PubMed.
- W. L. Steffen and G. J. Palenik, Acta Crystallogr., Sect. B, 1976, 32, 298 CrossRef.
- W. L. Steffen and G. J. Palenik, Inorg. Chem., 1977, 16, 1119 CrossRef CAS.
- J. F. Le Quarler, M. M. Borel and A. Leclaire, Acta Crystallogr., Sect. B, 1977, 33, 2299 CrossRef.
- R. E. Marsh and V. Schomaker, Inorg. Chem., 1979, 18, 2331 CrossRef CAS.
- E. M. Cameron, W. E. Louch, T. S. Cameron and O. Knop, Z. Anorg. Allg. Chem., 1998, 624, 1629 CrossRef CAS.
- Y. Cui, D. Long, W. Chen and J. Huang, Acta Crystallogr., Sect. C, 1998, 54, 1605 CrossRef.
- C. A. Grapperhaus, T. Tuntulani, J. H. Reibenspiess and M. Y. Darensbourg, Inorg. Chem., 1998, 37, 4052 CrossRef CAS PubMed.
- J. Qin, N. Su, C. Dai, C. Yang, D. Liu, M. W. Day, B. Wu and C. Chen, Polyhedron, 1999, 18, 3461 CrossRef CAS.
- A. L. Spek, PLATON-94, University of Utrecht, The Netherlands, 1994 Search PubMed.
- H. v. Paulus, Z. Anorg. Allg. Chem., 1969, 369, 38 CrossRef CAS.
- R. D. Bailey and W. T. Pennington, Polyhedron, 1997, 16, 417 CrossRef CAS.
- J. Pickardt and B. Staub, Z. Naturforsch., B: Chem. Sci., 1997, 52, 1456 CAS.
- R. D. Bailey, L. L. Hook and W. T. Pennington, Chem. Commun., 1998, 1181 RSC.
- R. Taylor and O. Kennard, J. Am. Chem. Soc., 1982, 104, 5063 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
- S. S. Batsanov, Izv. Akad. Nauk, Ser. Khim., 1995, 24 Search PubMed.
- S. C. Nyburg and C. H. Faerman, Acta Crystallogr., Sect. B, 1985, 41, 274 CrossRef.
- D. C. Palmer, CrystalMaker: Interactive Crystallography for MacOS, CrystalMaker Software, Oxford, 1998 Search PubMed.
- A. Gavezzotti, J. Am. Chem. Soc., 1983, 105, 5220 CrossRef CAS.
- International Tables for Crystallography, Vol. A, ed. T. Hahn, Reidel, Dordrecht, The Netherlands, 1983 Search PubMed.
- G. R. Desiraju and R. Parthasarathy, J. Am. Chem. Soc., 1989, 111, 8725 CrossRef CAS.
- C. Hu and U. Englert, to be published.
| This journal is © The Royal Society of Chemistry 2001 |
