The chair conformation of C-methylcalix[4]resorcinarene in a novel, stepped, supramolecular framework
Bao-Qing
Ma
,
Yuegang
Zhang
and
Philip
Coppens
*
Department of Chemistry, State University of New York at Buffalo, Buffalo, NY 14260-3000, USA. E-mail: coppens@acsu.buffalo.edu
Abstract
The previously unobserved chair conformation of C-methylcalix[4]resorcinarene has been found in three different supramolecular solids, prepared by hydrothermal synthesis. The existence of the chair, crown and flattened cone conformations of C-methylcalix[4]resorcinarene demonstrates the dependence of the molecular conformation upon the templating molecules, and upon the specifics of the crystallization process. A novel, three-dimensional framework capable of including guest molecules is described.
In order to perform time-resolved photocrystallographic studies on molecules embedded within a periodic host framework,1,2 we have synthesized a number of new, supramolecular host–guest complexes.3C-Methylcalix[4]resorcinarene (CMCR) is a particularly suitable building block for such solids, as, together with ‘pillar’ molecules such as 4,4′-bipyridine (bipy), it can form solids with large cavities capable of including organic or inorganic guests.4,5 In a previous communication we reported a two-dimensional, brick-like framework incorporating decamethylruthenocene (DMR), with CMCR in a flattened cone (C2v) conformation, not previously observed for this molecule.3 We describe here three solids – CMCR·3bipy 1, CMCR·3bipy·benzophenone·2H2O 2, and CMCR·3bpe 3 [bpe⊕=⊕trans-bis(4-pyridyl)ethylene] – with a new framework in which the CMCR molecules have the (C2h) chair conformation.6 The rings located on the local two-fold axis bisecting the molecule are coplanar, while the other two rings are almost perpendicular to the plane containing the first two rings with a dihedral angle of 94.36(2)°. Although this conformation has been observed for other calix[4]resorcinarene derivatives,7–10 it has not been reported previously for CMCR.3,11 The formation of at least three conformations of one calix[4]resorcinarene demonstrates the flexibility of the molecular frame, together with the dependence of the conformation upon the templating molecules and also upon the specifics of the crystallization process.
The new solids are synthesized by hydrothermal methods,12 used widely in the preparation of quartz, zeolites and inorganic–organic hybrid open frameworks but less often for the synthesis of supramolecular organic systems.13 Low temperature X-ray diffraction analysis (Table 1) shows that 1 consists of a three-dimensional, hydrogen-bonded network. The four methyl groups of CMCR are in axial positions and have the rctt (reference–cis–trans–trans) arrangement of the C2h chair conformation, (Fig. 1) rather than the previously reported crown-shaped (C4v)14 or flattened cone (C2v) rccc conformations,3,15 the CMCR molecules being located on a crystallographic center of symmetry.
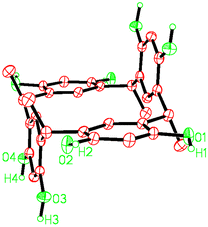 | ||
| Fig. 1 The CMCR chair conformation as observed in 1. | ||
| 1 | 2 | 3 | |
|---|---|---|---|
a All data were collected at T⊕=⊕90(1) K using a Bruker SMART1000 CCD with MoKα radiation (λ⊕=⊕0.71073 Å).
b Intensities were reduced using the SAINT program; structures were solved by direct methods and refined by a full-matrix, least squares technique based on F![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 using SHELXL97.
c Click b103190m.txt for full crystallographic data (CCDC 158837–158839). 2 using SHELXL97.
c Click b103190m.txt for full crystallographic data (CCDC 158837–158839).
|
|||
| Empirical formula | C62H56N6O8 | C75H70N6O11 | C68H62N6O8 |
| Crystal dimensions/mm | 0.30⊕×⊕0.18⊕×⊕0.10 | 0.32⊕×⊕0.12⊕×⊕0.10 | 0.40⊕×⊕0.32⊕×⊕0.06 |
| M | 1013.13 | 1231.37 | 1091.24 |
| Crystal system | Triclinic | Triclinic | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P1 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 9.8060(6) | 9.9541(14) | 10.7187(4) |
| b/Å | 10.8427(6) | 11.5239(16) | 11.1242(4) |
| c/Å | 13.0429(8) | 14.3145(14) | 13.1204(5) |
| α/° | 74.672(2) | 82.333(5) | 110.452(1) |
| β/° | 73.633(2) | 74.456(4) | 105.252(1) |
| γ/° | 89.016(2) | 80.836(3) | 96.896(1) |
| U/Å3 | 1280.78(13) | 1554.6(3) | 1374.76(9) |
| Z | 1 | 1 | 1 |
| D c/Mg m−3 | 1.314 | 1.315 | 1.318 |
| μ(Mo-Kα)/mm−1 | 0.088 | 0.089 | 0.087 |
| F(000) | 534 | 650 | 576 |
| GoF | 0.951 | 0.839 | 1.011 |
| Number of reflections (unique) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 555 (6214) 555 (6214) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198 (12 198 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553) 553) |
9578 (6202) |
| R int | 0.0534 | 0.0474 | 0.0318 |
| Number of observed reflections [I⊕>⊕2σ(I)] | 3990 | 6351 | 4282 |
| Number of refined parameters | 456 | 1011 | 495 |
| R 1 | 0.0550 | 0.0532 | 0.0483 |
| wR 2 | 0.1378 | 0.0956 | 0.1220 |
Adjacent CMCR molecules are connected into infinite columns parallel to the crystallographic [010] direction through two, center-of-symmetry-related, phenoxyl O–H⋯O hydrogen bonds per molecular pair [O1⋯O3a⊕=⊕2.7071(7) Å, O1–H1⋯O3a⊕=⊕163.5(11)°; a⊕=⊕−x⊕+⊕1, −y⊕+⊕2, −z⊕+⊕1]. As shown in Fig. 2, O–H⋯N hydrogen bonds between the axial phenoxyl groups and bipyridine molecules link the columns into stair-like sheets parallel to the (011) plane [O4⋯N2⊕=⊕2.7529(9) Å, O4–H4⋯N2⊕=⊕176.2(11)°; O3⋯N1b⊕=⊕2.6712(9) Å, O3–H3⋯N1b⊕=⊕167.7(12)°; b⊕=⊕−x, −y⊕+⊕2, −z]. The sheets are connected with each other by a second set of bipyridyl molecules through O–H⋯N hydrogen bonds giving rise to a three-dimensional network [O2⋯N3c⊕=⊕2.8885(10) Å, O2–H2⋯N3c⊕=⊕174.0(8)°; c⊕=⊕x, y⊕+⊕1, z] (Fig. 3).
![The stepped sheet of 1, consisting of hydrogen-bonded CMCR and bipyridine molecules, (a) viewed along the [100] direction, (b) viewed along [010]. Click image or here to access a 3D representation.](/image/article/2001/CE/b103190m/b103190m-f2.gif) | ||
| Fig. 2 The stepped sheet of 1, consisting of hydrogen-bonded CMCR and bipyridine molecules, (a) viewed along the [100] direction, (b) viewed along [010]. Click image or 2.htm to access a 3D representation. | ||
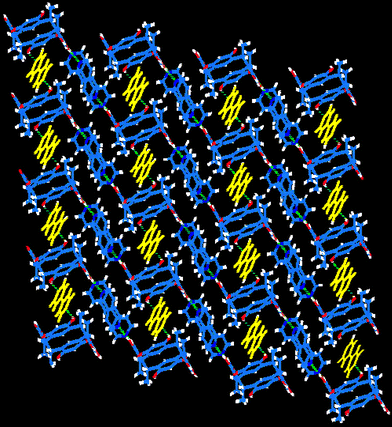 | ||
| Fig. 3 The three-dimensional network of 1. One sheet is shown. Bipyridine molecules connecting the sheets are shown in yellow. Click image or 3.htm to access a 3D representation. | ||
Three different kinds of host structures formed by CMCR and bipyridine which are capable of including guest molecules have been described in the literature: a 0D carcerand-like capsule;16 a 1D wave-like polymer;5 and a 2D brick-wall-like sheet.3 The stepped network can similarly accommodate guest molecules, as demonstrated by the structure of CMCR·3bipy·benzophenone·2H2O 2, in which CMCR also adopts a chair conformation. As shown in Fig. 4, in crystals of 2 the CMCR molecules are again linked into hydrogen-bonded columns, oriented parallel to the [100] direction, with water molecules interspersed between the CMCRs being part of the columns. The columns are connected into stepped sheets by bipyridine molecules, as in 1, with large spaces remaining between dimeric bipyridine units. A second set of bipyridine molecules connects the sheets into a 3D network similar to that of 1 such that infinite channels are formed by adjacent cavities in successive sheets, the guest benzophenone molecules being located within the channels (Fig. 4).
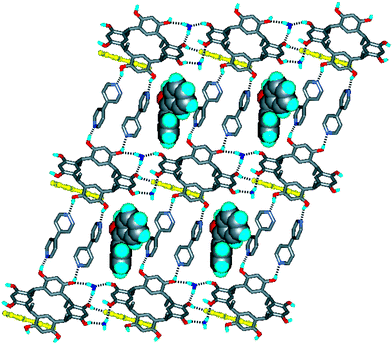 | ||
| Fig. 4 The three-dimensional, hydrogen-bonded framework of 2, formed by CMCR, bipyridine and water molecules, showing the guest benzophenone molecules. The bipyridine molecules linking the stepped sheets are shown in yellow. Click image or 4.htm to access a 3D representation. | ||
The shape of the guest molecules is affected by the host matrix: the twist angles between the C(C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O)C plane and the two phenyl ring planes of benzophenone are 29.3(2) and 16.7(3)°, significantly different from those in neat benzophenone crystals (α form: 31.0 and 28.7°,17 30.8 and 30.0°;18
β form: 30.8 and 46.8°19). The carbonyl oxygen atom of benzophenone forms a hydrogen bond with one of the water molecules.
O)C plane and the two phenyl ring planes of benzophenone are 29.3(2) and 16.7(3)°, significantly different from those in neat benzophenone crystals (α form: 31.0 and 28.7°,17 30.8 and 30.0°;18
β form: 30.8 and 46.8°19). The carbonyl oxygen atom of benzophenone forms a hydrogen bond with one of the water molecules.
In photochemical studies of host–guest complexes of benzil and decamethylruthenocene we have found considerable differences between triplet state lifetimes of the same guest species within different host frameworks, illustrating the effect of the environment upon the photochemical properties.20 (The relation between the molecular conformation of benzophenone and the triplet state lifetimes is to be explored in subsequent studies.) As expected, an appreciable dilution of the photoactive guest is achieved in the host–guest crystal 2 (1.07 vs. 6.64 mol dm−3 in the neat solid).17
When bipyridine is replaced by trans-bis(4-pyridyl)ethylene (bpe) in the reaction mixture used to prepare 1, a third solid, CMCR·3bpe 3, is formed. It is isostructural with 1, with CMCR again in the chair conformation, and has a stepped 3D framework (Fig. 5).
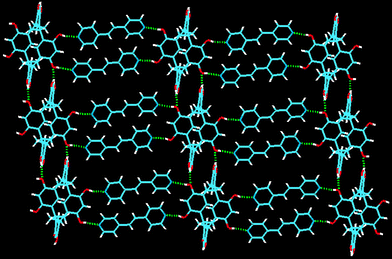 | ||
| Fig. 5 The two-dimensional stepped sheet of 3. Click image or 5.htm to access a 3D representation. | ||
In summary, the chair conformation for CMCR has been observed for the first time in several different phases prepared by hydrothermal synthesis, by which a novel, three-dimensional framework, capable of including guest molecules, has been synthesized.
Acknowledgements
Support of this work by the National Science Foundation (CHE9981864) and the Petroleum Research Fund of the American Chemical Society (PRF32638AC3) is gratefully acknowledged.References
- P. Coppens, G. Wu, A. Volkov, Y. Abramov, Y. Zhang, W. K. Fullagar and L. Ribaud, Trans. Am. Crystallogr. Assoc., 1999, 34, 51 CAS.
- P. Coppens, Synchrotron Radiat. News, 1997, 10, 26 CrossRef.
- Y. Zhang, C. D. Kim and P. Coppens, Chem. Commun., 2000, 2299 RSC.
- L. R. MacGillivray, J. L. Reid and J. Ripmeester, CrystEngComm, 1999, 1 Search PubMed.
- L. R. MacGillivray, H. A. Spinney, J. L. Reid and J. Ripmeester, Chem. Commun., 2000, 517 RSC.
- A. G. S. Högberg, J. Am. Chem. Soc., 1980, 102, 6046 CrossRef.
- A. Shivanyuk, E. F. Paulus, V. Bohmer and W. Vogt, Angew. Chem., Int. Ed. Engl., 1997, 36, 1301 CrossRef CAS.
- G. Rumboldt, V. Bohmer, B. Botta and E. F. Paulus, J. Org. Chem., 1998, 63, 9618 CrossRef CAS.
- L. M. Tunstad, J. A. Tucker, E. Dalcanale, J. Weiser, J. A. Bryant, J. C. Sherman, R. G. Helgeson, C. B. Knobler and D. C. Cram, J. Org. Chem., 1989, 54, 1505 CrossRef.
- O. Middel, W. Verboom, R. Hulst, H. Kooijman, A. L. Spek and D. N. Reinhoudt, J. Org. Chem., 1998, 63, 8259 CrossRef CAS.
- M. Nissinen, E. Wegelius, D. Falábu and K. Rissanen, CrystEngComm, 2000, 28 Search PubMed.
- Crystals of 1 were prepared as follows: C-methylcalix[4]resorcinarene (0.025 mmol), bipyridine (0.05 mmol) and benzil (0.05 mmol) were mixed with 4 ml of water and sealed in a heavy-walled Pyrex glass tube (ca. 6 ml). The mixture was maintained at 140
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 1 day in an oven and subsequently
cooled to room temperature at a rate of 20
°C for 1 day in an oven and subsequently
cooled to room temperature at a rate of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C per day. Colorless, plate-like crystals were collected. The benzil molecules are not incorporated into crystals of 1. However, different crystals within the same batch, to be described in a subsequent publication, did contain benzil. Crystals of 2 were obtained by a similar procedure in which benzil was replaced by benzophenone: C-methylcalix[4]resorcinarene (0.025 mmol), bipyridine (0.05 mmol) and benzophenone (0.05 mmol) were mixed with 4 ml of water and sealed in a heavy-walled Pyrex glass tube (ca. 6 ml). Again, the tube was maintained at 140
°C per day. Colorless, plate-like crystals were collected. The benzil molecules are not incorporated into crystals of 1. However, different crystals within the same batch, to be described in a subsequent publication, did contain benzil. Crystals of 2 were obtained by a similar procedure in which benzil was replaced by benzophenone: C-methylcalix[4]resorcinarene (0.025 mmol), bipyridine (0.05 mmol) and benzophenone (0.05 mmol) were mixed with 4 ml of water and sealed in a heavy-walled Pyrex glass tube (ca. 6 ml). Again, the tube was maintained at 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 1 day in an oven, then cooled to room temperature at a rate of 20
°C for 1 day in an oven, then cooled to room temperature at a rate of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C per day. Light yellow, plate-like crystals were collected..
°C per day. Light yellow, plate-like crystals were collected.. - Rao and coworkers used hydrothermal synthesis to prepare the 1∶1 organic adduct of cyanuric acid and melamine: A. Ranganathan, V. R. Pedireddi and C. N. R. Rao, J. Am. Chem. Soc., 1999, 121, 1752 CrossRef CAS ; see also: S. M.-F. Lo, S. S.-Y. Chui, L.-Y. Shek, Z. Lin, X. X. Zhang, G.-H. Wen and I. D. Williams, J. Am. Chem. Soc., 2000, 122, 6293 CrossRef.
- L. R. MacGillivray and J. L. Atwood, J. Am. Chem. Soc., 1997, 119, 6931 CrossRef CAS.
- L. R. MacGillivray and J. L. Atwood, Supramol. Chem., 2000, 11, 293 CrossRef CAS.
- L. R. MacGillivray, P. R. Diamente, J. L. Reid and J. Ripmeester, Chem. Commun., 2000, 359 RSC.
- G. M. Lobanova, Kristallografiya, 1968, 13, 984 CAS.
- E. B. Fleischer, N. Sung and S. Hawkinson, J. Phys. Chem., 1968, 72, 4311 CrossRef CAS.
- H. Kutzke, H. Klapper, R. B. Hammond and K. J. Roberts, Acta Crystallogr., Sect. B, 2000, 56, 486 CrossRef.
- G. Wu, C. Kim, Y. Zhang and P. Coppens, unpublished results.
| This journal is © The Royal Society of Chemistry 2001 |
