Crystal packing motifs for mixed oxalate/phenanthroline metal complexes
Vanessa
Russell
,
Donald
Craig
,
Marcia
Scudder
and
Ian
Dance
*
School of Chemistry, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: I.Dance@unsw.edu.au
Abstract
The preparations and crystal structures of (Ph4P)[Cr(phen)(ox)2](H2O) 1, [Co(phen)2(ox)](BF4) 2 and [Co(phen)2(ox)]I(H2O)2(EtOH)0.53 are described (phen⊕=⊕1,10-phenanthroline, ox⊕=⊕oxalate), and their crystal packing analysed in terms of the crystal supramolecular motifs and multiple aryl embraces. The crystal packing of 1 is determined by embrace motifs involving both the Ph4P+ cation and the anion [Cr(phen)(ox)2]−, principally an eight component concerted embrace (Ph4P+)·{[Cr(phen)(ox)2]−}2·(Ph4P+) comprised of three offset face-to-face (OFF) and six edge-to-face (EF) primary motifs. Complex 2 is dominated by well developed OFF-chain motifs for the phen ligands, reinforced by multiple C–H⋯O hydrogen bonds, to form a strongly hydrophobic lattice crystallising from water. Structure 3 crystallises with an efficient three-dimensional net using all three ligands of [Co(phen)2(ox)]+ in primary embrace motifs, with separate domains for the hydrogen bonded solvent molecules. The analyses identify crystal packing principles, especially the favourability of OFF phen embraces, the segregation of hydrophobic M(phen)n domains, and the prevalence of C–H⋯O hydrogen bonds involving oxalate or water. Predictions about crystal packing are made.
Introduction
In previous papers we have described the supramolecular motifs that occur in crystals of metal complexes containing the ligands 2,2′-bipyridine (bipy) and 2,2′:6′,2″-terpyridine (terpy). The preferred motif for complexes [M(bipy)3]z+ is the sixfold aryl embrace (6AE) comprised of a concerted cycle of six edge-to-face (EF) primary motifs. The crystal supramolecular motif for these complexes is a linear chain in which each [M(bipy)3]z+ forms two 6AEs.1 The dominant crystal supramolecular motif for [M(terpy)2]z+ and related complexes is a square two-dimensional net in which each complex is involved in four parallel fourfold aryl embraces (P4AE) with neighbouring complexes.2–4This investigation has been extended to metal complexes with the 1,10-phenanthroline (phen) ligand, and in an earlier paper5 we reported an investigation and survey of the M(phen)n (n⊕=⊕1, 2, 3) complexes recorded in the Cambridge Structural Database (CSD).6,7 Metal complexes of this planar aromatic ligand take part in both EF and OFF primary interactions (see Fig. 1), but with a clear preference for OFF motifs, consistent with the large surface area of the ligand (relative to bipy). The parallel fourfold aryl embrace [P4AE, see Fig. 1(d)] is also a strong feature of crystalline M(phen)2 and M(phen)3 complexes, as is the double edge-to-face (EF)2 [see Fig. 1(c)]. Extended motifs in one and two dimensions are propagated mainly by OFF primary motifs, and one three-dimensional net was identified.5
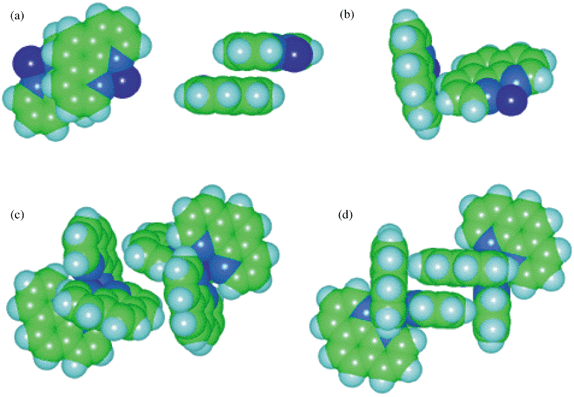 | ||
| Fig. 1 (a) Two views of the offset face-to-face (OFF) interaction, (b) the edge-to-face (EF) interaction between a pair of coordinated phenanthroline ligands, (c) the double edge-to-face, (EF)2, and (d) the parallel fourfold aryl embrace, P4AE, formed between a pair of M(phen)2X complexes (here X⊕=⊕phen). | ||
A current experimental program involves the crystallisation of metal complexes containing phen ligands, exploring further the crystal supramolecular motifs. One general objective is to be able to predict crystal packing, and then to design and engineer crystals which incorporate specified packing features. In order to achieve this objective it is necessary to understand the energies of the supramolecular motifs5 and to understand the synergies and antagonisms between phen-based motifs and those of other components, and also the competitions between different supramolecular forces. In this paper we consider complexes containing phen ligands and non-bridging oxalate (ox) ligands, and report the crystal structures and packing of three compounds, (Ph4P)[Cr(phen)(ox)2](H2O) 1, [Co(phen)2(ox)](BF4) 2, and [Co(phen)2(ox)]I(H2O)2(EtOH)0.53. The bidentate oxalate ligand is a hydrogen bond acceptor and can participate in hydrophilic interactions, in contrast to the distinctly hydrophobic character of M(phen)n embraces. Compound 1 includes only one phen ligand but also a Ph4P+ cation, which itself is well known to participate in multiple phenyl embraces which can extend in one, two and three dimensions.8–12 Thus these compounds present competing possibilities, which are analysed in this paper.
A considerable number of mixed ligand phen–ox metal complexes is known,13,14 but the only structures reported with coordinates in the CSD are a diastereomer of [Cr(phen)(ox)2]−,15 (Ph4As)[Cr(phen)(ox)2]H2O (reported16 while our work was in progress), and [Cu(phen)(ox)(H2O)]H2O.17,18
These oxalate complexes are distinct from another well known class in which oxalate bridges generate two- and three-dimensional nets.19–21
Experimental
Preparation and crystallisation
X-Ray crystallography
Reflection data were measured using an Enraf-Nonius CAD-4 diffractometer in θ/2θ scan mode using graphite monochromated molybdenum radiation (λ⊕=⊕0.7107 Å) for 1 and 2 and copper radiation (λ⊕=⊕1.5418 Å) for 3. Data were corrected for absorption, using the analytical method of de Meulenaer and Tompa,22 and for any decomposition. Reflections with I⊕>⊕3σ(I) were considered observed. Structures were determined by direct phasing and Fourier methods. Hydrogen atoms in all structures were included in calculated positions, except for those on water molecules. For 1 the phenyl rings of the cation were refined as rigid groups of mm2 symmetry. BF4− ions in 2 were also refined as rigid groups, with Td symmetry. The metal atoms, oxalate ligands, iodide, water oxygen and ethanol carbon and oxygen were refined anisotropically. Phenanthroline ligands and phenyl rings were refined with their thermal motion described by 12 parameter TL groups, whereas the BF4− ions were refined using a 15 parameter TLX thermal group. Reflection weights used were 1/σ2(Fo), with σ(Fo) being derived from σ(Io)⊕=⊕[σ2(Io)⊕+⊕(0.04Io)2]1/2. The weighted residual is defined as Rw⊕=⊕(ΣwΔ2/ΣwFo2)1/2. Atomic scattering factors and anomalous dispersion parameters were from International Tables for X-Ray Crystallography.23 Structure solution was by SIR9224 and refinement for all structures used RAELS.25 Details of the data collection and refinement are given in Table 1.| Parameter | 1 | 2 | 3 |
|---|---|---|---|
| a Click b102474b.txt for full crystallographic data (CCDC 158792–158794). | |||
| Empirical formula | C40H30CrN2O9P | C26H16BCoF4N4O4 | C27H23CoIN4O6.5 |
| M | 765.7 | 594.2 | 693.3 |
| Crystal system | Triclinic | Monoclinic | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
C2/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 9.422(6) | 14.964(8) | 11.063(7) |
| b/Å | 14.056(10) | 13.225(5) | 11.379(6) |
| c/Å | 14.681(11) | 12.718(7) | 11.990(6) |
| α/° | 103.49(4) | 90 | 91.34(4) |
| β/° | 99.84(4) | 107.51(2) | 113.48(3) |
| γ/° | 106.66(4) | 90 | 104.59(3) |
| V/Å3 | 1751(2) | 2400(2) | 1327(1) |
| Z | 2 | 4 | 2 |
| T/K | 294 | 294 | 294 |
| D c/g cm−3 | 1.45 | 1.64 | 1.74 |
| μ(Mo)/mm−1 | 0.421 | 0.784 | 14.99 (Cu) |
| 2θmax/° | 46 | 50 | 100 |
| Crystal decay (%) | None | 37 | 47 |
| Min. transmission factor | 0.91 | 0.92 | 0.10 |
| Max. transmission factor | 0.96 | 0.93 | 0.38 |
| Unique reflections | 4565 | 2102 | 2706 |
| Observed reflections | 2768 | 1012 | 1933 |
| R merge | 0.014 | 0.062 | 0.069 |
| R | 0.042 | 0.052 | 0.058 |
| R w | 0.052 | 0.061 | 0.073 |
Results
Intramolecular geometries in all complexes are unexceptional.Crystal packing of (Ph4P)[Cr(phen)(ox)2](H2O), 1
This compound is, to our knowledge, the only one that contains both phen and Ph4P+ as potentially embracing entities. Recently, after completion of our work, the crystal structure of the isomorphous compound (Ph4As)[Cr(phen)(ox)2](H2O) was reported,16 but its crystal supramolecularity was not analysed completely.The overall crystal packing of 1, in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , is shown in Fig. 2(a). There are distinct domains for the Ph4P+ ions and the [Cr(phen)(ox)2]− complexes, and several important supramolecular motifs. Fig. 2(b) shows that there are well developed chains of Ph4P+ ions along the z-direction, together with a good OFF interaction between the phen ligands of the [Cr(phen)(ox)2]− complexes which also occur in chains. The chain of Ph4P+ ions is comprised of alternating 6PE and OFF motifs, as illustrated in Fig. 3. This (·6PE··OFF·)∞ chain
is a modification of the commonly observed ZZI6PE chain9 which is (·6PE··6PE·)∞. The (·6PE··OFF·)∞ sequence allows the chain of Ph4P+ ions to be straighter than the ZZI6PE, and to have a longer repeat distance of 14.7 Å compared with ca. 10 Å for the ZZI6PE. There are no significant interactions between adjacent chains of cations within the cation domain.
, is shown in Fig. 2(a). There are distinct domains for the Ph4P+ ions and the [Cr(phen)(ox)2]− complexes, and several important supramolecular motifs. Fig. 2(b) shows that there are well developed chains of Ph4P+ ions along the z-direction, together with a good OFF interaction between the phen ligands of the [Cr(phen)(ox)2]− complexes which also occur in chains. The chain of Ph4P+ ions is comprised of alternating 6PE and OFF motifs, as illustrated in Fig. 3. This (·6PE··OFF·)∞ chain
is a modification of the commonly observed ZZI6PE chain9 which is (·6PE··6PE·)∞. The (·6PE··OFF·)∞ sequence allows the chain of Ph4P+ ions to be straighter than the ZZI6PE, and to have a longer repeat distance of 14.7 Å compared with ca. 10 Å for the ZZI6PE. There are no significant interactions between adjacent chains of cations within the cation domain.
, 1. The carbon atoms of the cations are coloured blue and those of the phen ligands coloured green, to emphasise the layering of cations and anions; oxalate carbon atoms are yellow in this and subsequent figures; water oxygen atoms are black. (a) The ·6PE··OFF··6PE··OFF· chain of Ph4P+ is horizontal. (b) View along the ·6PE··OFF··6PE··OFF· chain of Ph4P+ and parallel to the OFF motifs between phen ligands. Note the association of the water molecules (black) with the oxalate ends of the complexes. Click image or here to access a 3D representation.](/image/article/2001/CE/b102474b/b102474b-f2.gif) | ||
| Fig. 2 Crystal packing of (Ph4P)[Cr(phen)(ox)2](H2O), 1. The carbon atoms of the cations are coloured blue and those of the phen ligands coloured green, to emphasise the layering of cations and anions; oxalate carbon atoms are yellow in this and subsequent figures; water oxygen atoms are black. (a) The ·6PE··OFF··6PE··OFF· chain of Ph4P+ is horizontal. (b) View along the ·6PE··OFF··6PE··OFF· chain of Ph4P+ and parallel to the OFF motifs between phen ligands. Note the association of the water molecules (black) with the oxalate ends of the complexes. Click image or 2.htm to access a 3D representation. | ||
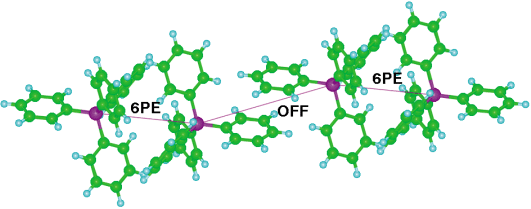 | ||
| Fig. 3 Cation chain in 1. 6PE alternate with OFF interactions. | ||
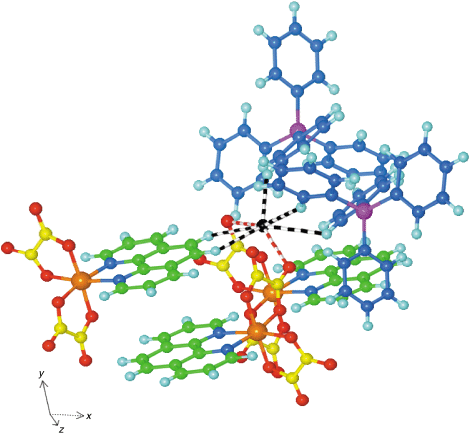
The OFF motif between adjacent [Cr(phen)(ox)2]− complexes does not stand alone, but is intimately involved in a larger multi-component embrace involving two [Cr(phen)(ox)2]− anions and two Ph4P+ cations from chains on opposite sides of the layer of anions. This (Ph4P+)·{[Cr(phen)(ox)2]−}2·(Ph4P+) embrace, pictured in Fig. 4, is centrosymmetric and involves two phenyl rings from each Ph4P+, and the phen ligand and one oxalate ligand from each [Cr(phen)(ox)2]−, a total of eight embracing components. Between these there are three OFF local motifs, and six EF local motifs. In addition to the phen⋯phen OFF motif across the centre of inversion (the two phen planes are separated by 3.4 Å), there is one between the outer surfaces of these phen ligands and one phenyl ring of each cation (the phen⋯phenyl rings are slightly inclined). There are two each of the EF interactions phen⊕⇒⊕ox, phen⊕⇒⊕phenyl, and phenyl⊕⇒⊕phen, marked on Fig. 4. This is an embrace involving oppositely charged ions, and therefore is more favourable electrostatically than the more commonly analysed embraces between same-charged ions.
![(Ph4P+)·{[Cr(phen)(ox)2]−}2·(Ph4P+) multiple embrace in 1: Cr orange, P purple, C (phen) green, C (phenyl) blue, C (oxalate) yellow, O red, H pale blue. This motif is centrosymmetric. (a) The three independent EF motifs are labelled: one is phen⊕⇒⊕ox, one phen⊕⇒⊕phenyl, one phenyl⊕⇒⊕phen, and another three are generated by the centre of inversion. The three OFF motifs are also marked. (b) View rotated from (a) by 90° around the horizontal axis, showing how (on the left side) the ox and phenyl sections can lie over the edge of the phen ligand.](/image/article/2001/CE/b102474b/b102474b-f4.gif) | ||
| Fig. 4 (Ph4P+)·{[Cr(phen)(ox)2]−}2·(Ph4P+) multiple embrace in 1: Cr orange, P purple, C (phen) green, C (phenyl) blue, C (oxalate) yellow, O red, H pale blue. This motif is centrosymmetric. (a) The three independent EF motifs are labelled: one is phen⊕⇒⊕ox, one phen⊕⇒⊕phenyl, one phenyl⊕⇒⊕phen, and another three are generated by the centre of inversion. The three OFF motifs are also marked. (b) View rotated from (a) by 90° around the horizontal axis, showing how (on the left side) the ox and phenyl sections can lie over the edge of the phen ligand. | ||
The water molecule in the crystal forms two O–H⋯O(oxalate) hydrogen bonded links between pairs of anions. As is evident in Fig. 2, the O atom of the water molecule lies almost within the phenyl domain of the Ph4P+ cations, and in fact is surrounded by five C–H bonds which come from the phenyl groups and the edge of the phen ligand. Details of the surroundings of the water molecule are presented in Fig. 5.
 | ||
| Fig. 5 Around the water molecule in 1 there are five C–H⋯O(water) contacts at < 3 Å (black and white candystripes), in addition to the two (water)O–H⋯O(oxalate) hydrogen bonds (red and white). | ||
This compound, containing four peripheral oxalate O atoms per anion, was crystallised from water and yet contains only one water molecule which, while linking as hydrogen bond donor to two oxalate O atoms, is located in a hydrophobic domain of the crystal. This illustrates our conclusion that 1 is a well packed, tightly embracing, largely hydrophobic crystal. We consider that the tight multiple embraces involving cations and anions, shown in Fig. 4, lead to the modification of the cation chain from the normal ·6PE··6PE··6PE· chain of the ZZI6PE to the ·6PE··OFF··6PE··OFF· chain observed. The attractive energies of all of the embraces involving phenyl, phenanthroline and oxalate are believed to exceed the hydrogen bonding energies of an alternative structure in which the oxalate ligands are fully hydrated.
Crystal packing of [Co(phen)2(ox)](BF4), 2
This compound crystallises in the monoclinic space group C2/c. The overall crystal packing is shown in Fig. 6. The [Co(phen)2(ox)]+ and the BF4− are located on the two-fold axes, and alternate along each two-fold axis as shown in Fig. 7. The BF4− ion nestles close to two phen ligands (see below) but is quite distant from the oxalate O atoms of the next cation. Adjacent [Co(phen)2(ox)]+⋯BF4−⋯[Co(phen)2(ox)]+⋯BF4− chains are positioned along the two-fold axes such that phen ligands from adjacent chains can approach each other and form OFF motifs, pictured in Fig. 8. These OFF motifs form well developed zig-zag OFF chains, and represent a general one-dimensional motif for M(phen)2 complexes, as we have described previously.5 In the present instance of zig-zag OFF chains involving M(phen)2 complexes where the third chelate ligand is oxalate, there are multiple weak C–H⋯O hydrogen bonds reinforcing the chain, as illustrated in Fig. 8.
2, showing how the cation⋯anion chains parallel to y are aligned to allow phen ligands of adjacent chains to form phen···phen motifs in the x direction. Co dark blue, C (phen) green, C (ox) yellow, N blue, O red, B magenta, F purple. Click image or here to access a 3D representation.](/image/article/2001/CE/b102474b/b102474b-f6.gif) | ||
| Fig. 6 Crystal packing in [Co(phen)2(ox)](BF4) 2, showing how the cation⋯anion chains parallel to y are aligned to allow phen ligands of adjacent chains to form phen···phen motifs in the x direction. Co dark blue, C (phen) green, C (ox) yellow, N blue, O red, B magenta, F purple. Click image or 6.htm to access a 3D representation. | ||

2, showing the alternation of [Co(phen)2(ox)]+ and BF4− ions along a two-fold axis parallel to b. The shortest F⋯O distance is 3.99 Å.](/image/article/2001/CE/b102474b/b102474b-f7.gif) | ||
| Fig. 7 Part of the crystal structure of [Co(phen)2(ox)](BF4) 2, showing the alternation of [Co(phen)2(ox)]+ and BF4− ions along a two-fold axis parallel to b. The shortest F⋯O distance is 3.99 Å. | ||
![Zig-zag OFF chain of [Co(phen)2(ox)]+ ions in 2: colours are as for Fig. 6. The chain runs along the ac diagonal of the cell. The multiple C–H⋯O(oxalate) interactions are marked as candystripes: the shortest of these is H⋯O⊕=⊕2.46 Å.](/image/article/2001/CE/b102474b/b102474b-f8.gif) | ||
| Fig. 8 Zig-zag OFF chain of [Co(phen)2(ox)]+ ions in 2: colours are as for Fig. 6. The chain runs along the ac diagonal of the cell. The multiple C–H⋯O(oxalate) interactions are marked as candystripes: the shortest of these is H⋯O⊕=⊕2.46 Å. | ||
There are additional C–H⋯O(oxalate) interactions, not shown in the figures, such that the uncoordinated O of oxalate is bound by three C–H bonds of two adjacent phen ligands.
Again it is significant that this crystal does not contain water from its crystallisation solution: the oxalate and the BF4− anions often favour the inclusion of water by providing acceptor sites for O–H⋯O and O–H⋯F hydrogen bonding. It is clear that there is efficient alternative packing in this crystal, expressed in the OFF motifs, the partial enclosure of the BF4−, and an effective network of weaker C–H⋯O hydrogen bonds.
Crystal packing of [Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3
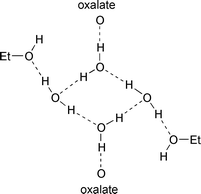
This compound contains the same cation as 2, with a single monatomic anion, but now a considerable amount of hydroxylic solvent is included in the crystal. The crystal (space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) is comprised of a three-dimensional net of embrace motifs between [Co(phen)2(ox)]+ complexes, with each complex engaging in five motifs using all three ligands. This net of embraces, pictured in Fig. 9, has some similarity with the diamondoid net in the sense that there are approximately hexagonal channels passing through it, but is clearly different in having five connections at each complex centre. The channels are occupied by chains comprised of (disordered) ethanol molecules and water molecules, which form hydrogen bonded cycles linking to oxalate ligands:
) is comprised of a three-dimensional net of embrace motifs between [Co(phen)2(ox)]+ complexes, with each complex engaging in five motifs using all three ligands. This net of embraces, pictured in Fig. 9, has some similarity with the diamondoid net in the sense that there are approximately hexagonal channels passing through it, but is clearly different in having five connections at each complex centre. The channels are occupied by chains comprised of (disordered) ethanol molecules and water molecules, which form hydrogen bonded cycles linking to oxalate ligands: ![Network of embrace motifs in the crystal structure of [Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3, marked as black rods between Co atoms. The five unique embrace motifs are identified by their Co⋯Co distances marked. The chains of hydrogen bonded ethanol (disordered) and water molecules occupy the channels in this net. O red, ethyl C green.](/image/article/2001/CE/b102474b/b102474b-f9.gif) | ||
| Fig. 9 Network of embrace motifs in the crystal structure of [Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3, marked as black rods between Co atoms. The five unique embrace motifs are identified by their Co⋯Co distances marked. The chains of hydrogen bonded ethanol (disordered) and water molecules occupy the channels in this net. O red, ethyl C green. | ||
The structure is a three-dimensional hydrophobic net, penetrated by hydrophilic domains distributed along channels within the hydrophobic net. The iodide ion is located within the phen embrace region of the lattice. The ethyl groups of the ethanol lie between pairs of parallel phen ligands. Click 9.htm to access a 3D representation.
In Fig. 9 the five embrace motifs in this structure are identified by their Co⋯Co distances. The 8.82 and 8.71 Å motifs are centrosymmetric OFF phen⋯phen connections, shown in Fig. 10, and include C–H⋯O(oxalate) hydrogen bonds. The 8.37 Å and 9.45 Å motifs are double edge-to-face (EF)2 phen···phen embraces,5 illustrated in Fig. 11. One of these centrosymmetric (EF)2 motifs has two I− ions associated with two of the phen ligands, in similar fashion to the association of polyiodide ions with [Cu(phen)2I]+ and [Fe(phen)3]2+ complexes.26–30
![The two centrosymmetric OFF phen···phen motifs in [Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3. Candystripes are C–H⋯O hydrogen bonds.](/image/article/2001/CE/b102474b/b102474b-f10.gif) | ||
| Fig. 10 The two centrosymmetric OFF phen···phen motifs in [Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3. Candystripes are C–H⋯O hydrogen bonds. | ||
![The two centrosymmetric (EF)2 phen···phen motifs in [Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3, identified by their Co⋯Co distances (see Fig. 9). In both motifs there is no overlap of the phen ligands nearer the centre of the motif, but an edge of each of these phen ligands is directed towards the face of the phen ligand on the other molecule. Two I− ions are associated with the 9.45 Å
(EF)2 motif, lying directly over the faces of the vertical phen ligands.](/image/article/2001/CE/b102474b/b102474b-f11.gif) | ||
| Fig. 11 The two centrosymmetric (EF)2 phen···phen motifs in [Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3, identified by their Co⋯Co distances (see Fig. 9). In both motifs there is no overlap of the phen ligands nearer the centre of the motif, but an edge of each of these phen ligands is directed towards the face of the phen ligand on the other molecule. Two I− ions are associated with the 9.45 Å (EF)2 motif, lying directly over the faces of the vertical phen ligands. | ||
The preceding four phen···phen motifs effectively utilise all four faces of the two phen ligands per [Co(phen)2(ox)]+ complex. The fifth motif between the complexes involves two oxalate ligands, which form an OFF motif (Co⋯Co 7.61 Å), pictured in Fig. 12. A search of the Cambridge Structural Database reveals that the ox⋯ox motif shown in Fig. 12 occurs in 94 of the 318 structures containing non-bridging oxalate ligands.
![Centrosymmetric OFF motif between two oxalate ligands in [Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3. The shortest C⋯O distance is 3.1 Å. The oxalate ligands are offset so that the more negatively polarised O atoms approach the more positive C atoms.](/image/article/2001/CE/b102474b/b102474b-f12.gif) | ||
| Fig. 12 Centrosymmetric OFF motif between two oxalate ligands in [Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3. The shortest C⋯O distance is 3.1 Å. The oxalate ligands are offset so that the more negatively polarised O atoms approach the more positive C atoms. | ||
Discussion
The three compounds described here are quite different in composition, but all contain [M(phen)2(ox)]+ or [M(phen)(ox)2]−. There are three different packing arrangements, each of which can be well understood. We first summarise our understanding of these crystal packing arrangements, and then extract some relevant principles. Table 2 summarises the crystal supramolecular motifs in structures 1–3.| Structure | Primary phen···phen motifs | Secondary phen···phen motif | Extended phen···phen motif | Hydrophilic domain | C–H⋯O hydrogen bonds | |
|---|---|---|---|---|---|---|
| EF | OFF | (EF)2 | OFF chain | |||
| 1 | ✓ | ✓ | ||||
| 2 | ✓ | ✓ | ✓ | |||
| 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
(Ph4P)[Cr(phen)(ox)2](H2O), 1, is dominated by embrace motifs involving both the Ph4P+ cation and the [Cr(phen)(ox)2]− anion. The principal embrace involves eight components (4⊕×⊕Ph, 2⊕×⊕phen, 2⊕×⊕ox) of the assembly (Ph4P+)·{[Cr(phen)(ox)2]−}2·(Ph4P+), and nine primary OFF and EF motifs, as (OFF)3(EF)6. In addition to this the Ph4P+ cations retain a variant on the commonly occurring ZZI6PE chain.9 The tightly packed lattice is essentially hydrophobic, even though formed in water, and only one water molecule hydrogen bonds to the exposed oxalate O atoms and also provides the acceptor site for C–H⋯O hydrogen bonds. We note that the OFF stack of phen ligands, an extended motif that is common for M(phen) complexes,5 does not occur in 1, probably because the (OFF)3(EF)6 cation···anion embrace is more favourable.
Crystalline [Co(phen)2(ox)](BF4), 2, is anhydrous, apparently because there are well developed OFF-chain motifs for the phen ligands, reinforced by multiple C–H⋯O hydrogen bonds, providing a cavity which contains the BF4− counter-ion. We believe that this lattice packing is determined principally by the concerted OFF plus C–H⋯O motifs linking [Co(phen)2(ox)]+.
[Co(phen)2(ox)]I(H2O)2(EtOH)0.5, 3, with the same cation as 2 and a smaller monatomic anion, crystallises with a three-dimensional net of embraces that is efficient because it uses all three ligands per [Co(phen)2(ox)]+ in primary embrace motifs. The I− associates with the face of a phen ligand, in a hydrophobic domain away from the hydrogen bonds. Ethanol and water form a cycle of hydrogen bonds with one oxalate O atom, with the ethyl chains directed from the hydrophilic domain into a hydrophobic region between phen ligands.
We raise a question about the different crystal supramolecularity of 2 and 3. They have the same [Co(phen)2(ox)]+ ion, which in both cases is involved in an evidently effective sequence of embraces, and both crystals contain a relatively small anion. This anion is not solvated but is in a hydrophobic region adjacent to phen ligands. Why then do 2 and 3 have different supramolecular motifs and crystal packing? Could each of 2 and 3 have crystallised with the crystal supramolecularity of the other? Are there unrecognised polymorphs for these compounds? Further experiments are needed because the crystallisation solvent system was different in the two experiments, with ethanol present only for 3.
The following general features and principles are noted:
Structures
1
,
2
and
3
are efficiently packed, with the only indications of looseness occurring where the ethyl chain is in a slightly larger cavity than needed in
3
.
All of the phen ligands in the complexes embrace fully, and by so doing form tight, hydrophobic domains.
The OFF motif is present in all cases, consistent with the statistics for this motif for all phen complexes in the CSD.5
Hydrogen bonding C–H⋯O occurs as a strong feature of all compounds.
The oxalate ligands are involved more in C–H⋯O hydrogen bonds than in solvated hydrophilic domains.
In all crystal packing analysis it is necessary to consider shape factors. Apart from Ph4P+ most of the other components in these crystals are relatively small and globular, and there is no indication that shape awkwardness is an issue in the crystal supramolecularity of these crystals.
Predictions
A goal of this research is the ability to predict, and then to design and engineer, the structures of molecular crystals with similar metal complex components. Some predictions about metal phenanthroline complexes were made in our earlier paper,5 and on the basis of the observations here it is possible to formulate working hypotheses to be tested with further crystallisations of metal complexes involving phenanthroline and related ligands.We predict that metal complexes with phen
ligands
and phen-related ligands will associate using the embraces described, and that they will form crystals (from protic media) with hydrophobic domains.
We predict that hydrophilic components of these crystals (such as counter-ions) will commonly be segregated from the embrace domains.
On the basis of the occurrence of related but different combinations of multiple aryl embraces for similar compounds (
cf.
2
and
3
), we expect that polymorphs (or substitutional polymorphs
31–33
) will appear during further crystallisation experiments.
We note that C–H⋯X hydrogen bonds
34
from phen ligands are a significant force in crystal packing (at least for X⊕=⊕O, Cl,
35
I
26–30
), and that the O (or similar) acceptors for these could be part of other ligands in the complex,
e.g.
[M(phen)
n
(oxo-edged-ligands)] (
n
⊕=⊕1, 2), or that they could be in associated counter-ions such as oxometallate anions.
Acknowledgements
This research is supported by the Australian Research Council. V. R. acknowledges the award of an Australian Postgraduate Award.References
- I. Dance and M. Scudder, J. Chem. Soc., Dalton Trans., 1998, 1341 RSC
.
- M. L. Scudder, H. A. Goodwin and I. G. Dance, New J. Chem., 1999, 23, 695 RSC
.
-
J. McMurtrie and I. G. Dance, to be published, 2001
.
- N. W. Alcock, P. R. Barker, J. M. Haider, M. J. Hannon, C. L. Painting, Z. Pikramenou, E. W. Plummer, K. Rissanen and P. Saarenketo, J. Chem. Soc., Dalton Trans., 2000, 1447 RSC
.
- V. M. Russell, M. L. Scudder and I. G. Dance, J. Chem. Soc., Dalton Trans., 2001, 789 RSC
.
- F. H. Allen, J. E. Davies, J. J. Galloy, O. Johnson, O. Kennard, C. F. Macrae and D. G. Watson, Chem. Inf. Comput. Sci., 1991, 31, 204 CrossRef
.
- F. H. Allen and O. Kennard, Chem. Des. Automat. News, 1993, 8, 131 Search PubMed
.
-
I. G. Dance, in Perspectives in Supramolecular Chemistry: The Crystal as a Supramolecular Entity,
ed. G. R. Desiraju, Wiley, Chichester, 1996, pp. 137–233 Search PubMed
.
- I. Dance and M. Scudder, J. Chem. Soc., Dalton Trans., 1996, 3755 RSC
.
- I. Dance and M. Scudder, Chem. Eur. J., 1996, 2, 481 CrossRef CAS PubMed
.
- M. Scudder and I. Dance, J. Chem. Soc., Dalton Trans., 1998, 3155 RSC
.
- M. Scudder and I. Dance, J. Chem. Soc., Dalton Trans., 1998, 3167 RSC
.
- J. A. Broomhead, Aust. J. Chem., 1961, 15, 228 CrossRef
.
- J. A. Broomhead, M. Dwyer and N. Kane-Maguire, Inorg. Chem., 1968, 7, 1388 CrossRef CAS
.
- H. Ohbo, H. Okazaki, K. Miyoshi and H. Yoneda, Bull. Chem. Soc. Jpn., 1983, 56, 1982 CrossRef CAS
.
- G. Marinescu, M. Andruh, R. Lescouëzec, M. C. Muñoz, J. C. Cano, F. Lloret and M. Julve, New J. Chem., 2000, 24, 527 RSC
.
- A. C. Fabretti, C. Franchini, P. Zannini and M. Di Vaira, Inorg. Chim. Acta, 1985, 105, 187 CrossRef
.
- C.-Y. Su, B. Kang and D.-K. Li, Chin. J. Struct. Chem. (Jiegou Huaxue), 1998, 17, 147 CAS
.
- M. Hernandez-Molina, F. Lloret, C. Ruiz-Perez and M. Julve, Inorg. Chem., 1998, 37, 4131 CrossRef CAS PubMed
.
- S. Decurtins and R. Pellaux, Comments Inorg. Chem., 1998, 20, 143 CrossRef CAS
.
-
V. M. Russell, D. C. Craig, M. L. Scudder and I. G. Dance, CrystEngComm, 2000, 3; http://www.rsc.org/ej/ce/2000/a909749j/index.htm Search PubMed
.
- J. de Meulenaer and H. Tompa, Acta Crystallogr., 1965, 19, 1014 CrossRef CAS
.
-
International Tables for X-Ray Crystallography, ed. J. A. Ibers and W. C. Hamilton, Kynoch Press, Birmingham, 1974, vol. 4 Search PubMed
.
- A. Altomare, M. C. Burla, M. Camalli, G. Cascarano, C. Giacovazzo, A. Guagliardi and G. J. Polidori, J. Appl. Crystallogr., 1994, 27, 435 Search PubMed
.
-
A. D. Rae, RAELS92, A Comprehensive Constrained Least Squares Refinement Program, Australian National University, Canberra, Australia, 1992 Search PubMed
.
-
C. Horn, B. F. Ali, I. G. Dance, M. L. Scudder and D. C. Craig, CrystEngComm, 2000, 2; http://www.rsc.org/ej/ce/2000/a910231k/index.htm Search PubMed
.
-
C. Horn, M. L. Scudder and I. G. Dance, CrystEngComm, 2000, 9;
http://www.rsc.org/ej/ce/2000/b001706j/index.htm Search PubMed
.
-
C. Horn, M. L. Scudder and I. G. Dance, CrystEngComm, 2000, 36; http://www.rsc.org/ej/ce/2000/B008751N/index.htm Search PubMed
.
-
C. Horn, M. L. Scudder and I. G. Dance, CrystEngComm, 2001, 1; http://www.rsc.org/ej/ce/2001/B008381J/index.htm Search PubMed
.
-
C. Horn, M. L. Scudder and I. G. Dance, CrystEngComm, 2001, 2;
http://www.rsc.org/ej/ce/2001/B008107H/index.htm Search PubMed
.
- D. Braga and F. Grepioni, Chem. Soc. Rev., 2000, 29, 229 RSC
.
-
C. Horn and I. G. Dance, 2001, to be published
.
-
P. A. W. Dean, M. L. Scudder, D. Craig and I. G. Dance, CrystEngComm, 2001, 22; http://www.rsc.org/ej/ce/2001/B102399N/index.htm Search PubMed
.
-
G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond in Structural Chemistry and Biology, Oxford University Press, Oxford, 1999 Search PubMed
.
-
F. Maharaj, V. M. Russell, M. L. Scudder, D. C. Craig and I. G. Dance, CrystEngComm, 2001, to be submitted Search PubMed
.
| This journal is © The Royal Society of Chemistry 2001 |